Midterm 2 Review
1/30
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
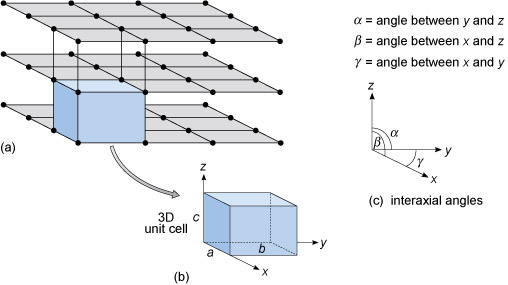
What does “Z” refer to when describing unit cells?
the number of times the chemical formula of a mineral repeats in its crystallographic unit cell
Birefringence
Also known as double refraction; seen in anisotropic minerals as interference colors. When light hits a crystal and splits into two rays. These rays travel in different directions and are polarized differently.
Difference between translational symmetry and point symmetry
Translational symmetry means sliding/shifting an object without rotating it and still seeing the same image. Point symmetry involves a shape appearing unchanged after a 180-degree rotation around a central point (rotational symmetry)
Primitive lattice vs. centered lattice
Primitive lattice only has points at the corners of the unit cell
Centered lattice has points at the corners and at center of the unit cell, the faces, or both
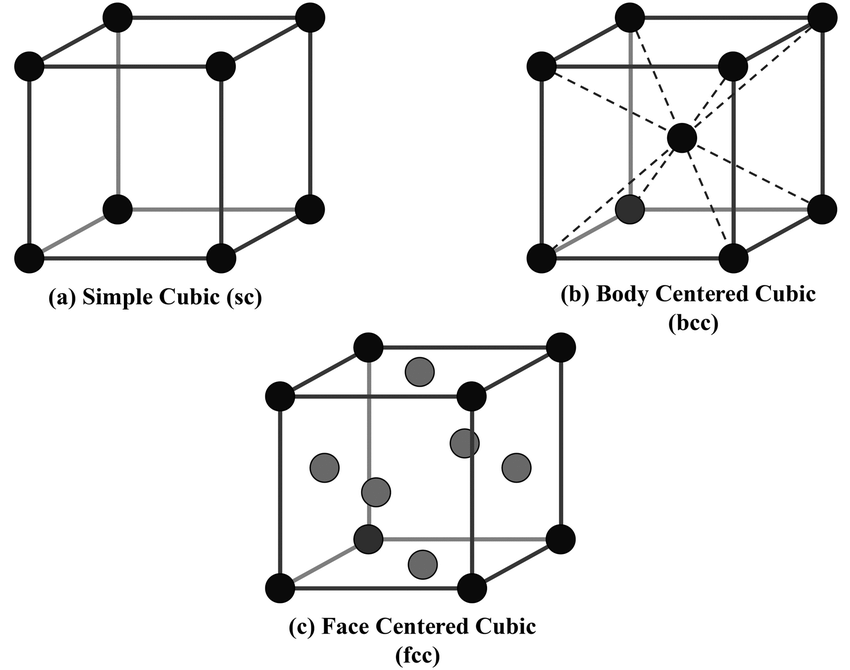
Why does the hexagonal system have an extra axis?
The hexagonal system has a 4th axis because it shows the 3 identical axes that are perpendicular to the vertical c-axis. 3 axes would be inadequate by themselves. The extra axis helps distinguish hexagonal crystals’ unique 6-fold symmetry
What is the difference between Bravais lattices, point groups, and space groups?
Bravais lattices describe periodic arrangements of points in space
Point groups focus on symmetry operations that leave at least one point fixed (32)
Space groups combine both translational and point symmetry to describe the complete symmetry of a crystal structure
How do we keep track of the faces and planes within crystals?
Law of constancy of interfacial angles. The angles between internal crystal faces are always the same in one type of crystal, even if external faces look different. Miller indices also mark faces by which axes they intercept
How are individual faces notated versus collections of faces (forms)? How are negative values indicated?
Individual faces are noted with one number (0 or 1). A collection of faces is indicated with 3 values {xxx}. A 1 means a face intersects an axis, while a 0 means it does not. For example, {011} means a face intersects 2 axes. Negative values are indicated with a bar over the number (bar1, bar2, etc).
What’s the difference between PPL and XPL?
PPL stands for plane polarized light, and XPL stands for cross polarized light. PPL is better for detecting physical characteristics in minerals/pleochroism. XPL is better for looking at interference colors and detecting anisotropic/isotropic minerals
Why does pleochroism occur?
Because the crystal absorbs a particular wavelength of light depending on the direction of vibration light passing through it. Observed with PPL
Miller index for an octahedron
{111}; faces intersect all 3 axes
Why do we see metastable minerals at the Earth’s surface?
Because some chemical reactions require activation energy to start, and at low temperatures, mineral reaction rates are slow, meaning it takes a very long time for them to reach a stable state (diamond).
What does a stable mineral at earth’s surface look like?
Stable minerals are those that remain unchanging despite their conditions; they have hard, shiny, well-defined crystals/grains, with distinct colors and textures, and are resistant to weathering (quartz). They exist in their lowest-possible energy state
What are the twin elements? Which types are produced by each?
Twin plane, twin center, and twin axis. Contact twins are generally caused by twin planes, where two minerals share a plane and touch, but do not interpenetrate (gypsum). Penetration twins are usually caused when two minerals share a center, in which they appear to grow into one another (staurolite). Cyclic twins may be caused by a twin axis, where minerals twin about one rotation axis, producing an identical, circular pattern radiating outwards (spinel)
How does light passing through a thin section (vs. nothing on the stage) change what we see through the microscope’s oculars (eyepiece)?
Light passing through the thin section allows us to see interference colors/pleochroism in the mineral as a result of birefringence and refractive index. The thin section redirects light through the upper polarizer.
What optical properties are related to the index of refraction? Describe how it is related to these properties.
The speed of the light, its wavelength, refraction, and dispersion are related to RI. A high refractive index means that light bends more when it passes through a material, causing it to slow down and its wavelength to shorten. Dispersion is also greater, since there is more scattering of colors due to how light bends
What is n? Define it in terms of velocities. Is nfast greater or less than nslow?
Refractive index; a measure of how much light slows down when passing through a medium. Ratio of light’s speed in a vacuum/its speed travelling through the material. In materials where light travels slower, n is higher, meaning nslow is greater than nfast
How is extinction observed through a microscope? Why does it occur 4 times in a full (360) rotation?
Extinction is seen through cross polarized light; it is when a mineral appears black under the microscope because it aligns with the polarizers (no light passes through). It occurs 4 times over a full rotation in anisotropic minerals because they refract light into 2 rays with different directions
Give examples of the types of extinction.
Inclined extinction; Long axis or cleavage plane is at an angle to the vibration direction. Triclinic and monoclinic minerals
Symmetrical extinction; extinction angles are the same across multiple cleavage planes/faces. High birefringence. Rhombohedral minerals (calcite)
Parallel extinction; mineral's long axis or cleavage plane is parallel to the vibration direction. Orthorhombic/hexagonal minerals
Undulatory extinction; mineral goes extinct at different, indeterminable points due to lack of cleavage and bent c axis (quartz).
Explain the difference between uniaxial and biaxial. Which systems are uniaxial? which are biaxial?
Uniaxial minerals operate around a single optic axis (the c axis), while biaxial minerals have 2 optic axes. Tetragonal and hexagonal crystals are uniaxial while orthorhombic, triclinic, and monoclinic crystals are biaxial
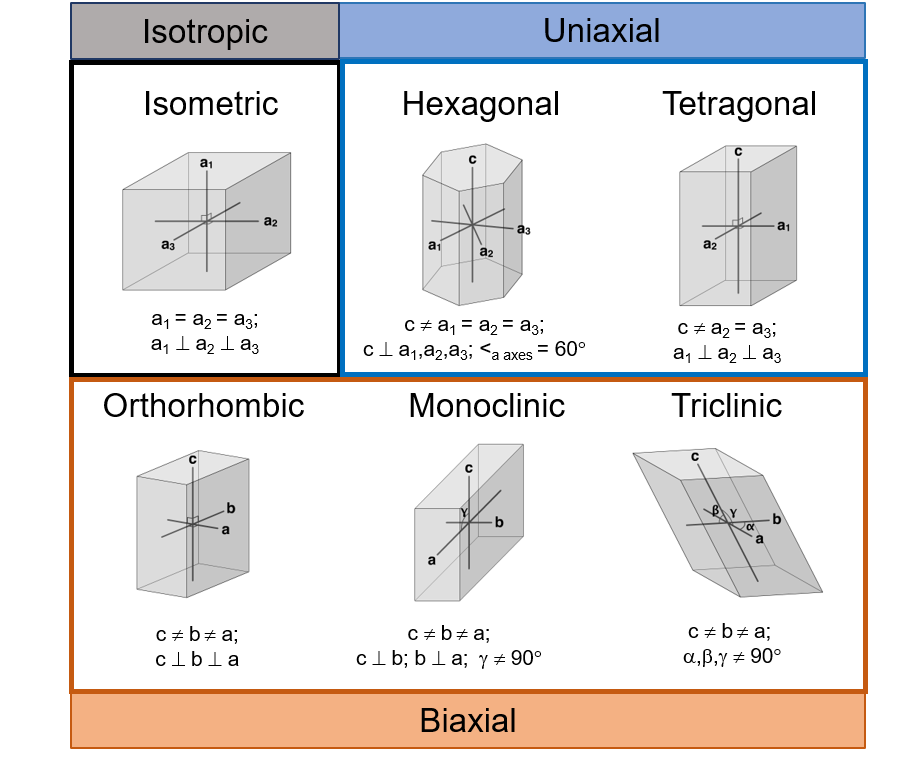
Why do biaxial minerals have two optic axes?
Because their refractive indices (α, β, and γ) are all different, resulting in two directions where light experiences no birefringence (double refraction)
What do you see in a petrographic microscope when looking down the optic axis of a mineral?
You are looking at an interference figure; it remains the same even as the mineral is rotated. It is used to determine optic sign and if a mineral is anisotropic or isotropic
Compare interference figures for anisotropic and isotropic minerals.
Isotropic minerals do not exhibit interference figures, appearing extinct across every rotation due to their lack of birefringence. Anisotropic minerals do exhibit birefringence due to high refractive index and so show distinct figures (crosses or curves) depending on orientation
Describe the birefringence (RI) equation; how is it related to retardation?
nfast - nslow; Retardation is a result of birefringence and the how much further the fast ray travels than the slow ray. It is calculated by the (thickness of the thin section) * (change in refractive index)
How are phase diagrams used to determine mineral stability and growth?
They are used to predict mineral changes/state transitions; using them, we can see when different states (solid or liquid) are stable, and when they are likely to occur in one or more minerals
Thermodynamically, what determines if a mineral is stable?
Gibbs free energy (energy level); it is a measure of the system's energy at a given temperature and pressure. A mineral is stable if it has the lowest energy compared to other possible phases under those conditions
What is the difference between fractional and equilibrium crystallization?
In equilibrium crystallization, crystals form and remain in contact with the melt, allowing the system to reach equilibrium; while in fractional crystallization, crystals are immediately removed from the melt (via floating or suspension), preventing the system from reaching equilibrium
Which crystal faces are the most prominent in minerals (slow or fast growing)? Why?
Slow growth faces are often more prominent because they allow for more complete and orderly atomic arrangement, resulting in well-defined and stable crystal faces
What controls the size and number of mineral grains that nucleate and grow?
Temperature and pressure are the biggest contributors to mineral grain growth. If a mineral cools very quickly, few crystals are likely to form. If it cools more slowly, however, it allows time for the mineral to grow larger crystals, and thus nucleation is higher
What is epitaxial nucleation?
When a new crystalline layer forms over an existing substrate crystal, and the grown layer's crystallography and orientation are determined by the substrate
What are the types of polymorphs? Give examples.
Order-disorder, reconstructive, and displacive.
In order-disorder, a mineral transitions between a more orderly and disorderly state in response to temperature/pressure. This change occurs without significantly changing crystal structure (sanidine to microcline feldspar).
In reconstructive polymorphism, there is extensive rearrangement of crystal structure, requiring the breaking/assembling of bonds. Requires high temp and pressure.
In displacive polymorphism, internal structure changes are small and easily reversible. Components are simply rotated/moved around (alpha to beta quartz).