Molecular geometry Organic chemistry
0.0(0)
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
Linear
-2 bonding
-0 non-bonding
-sp
-180 degrees

2
New cards
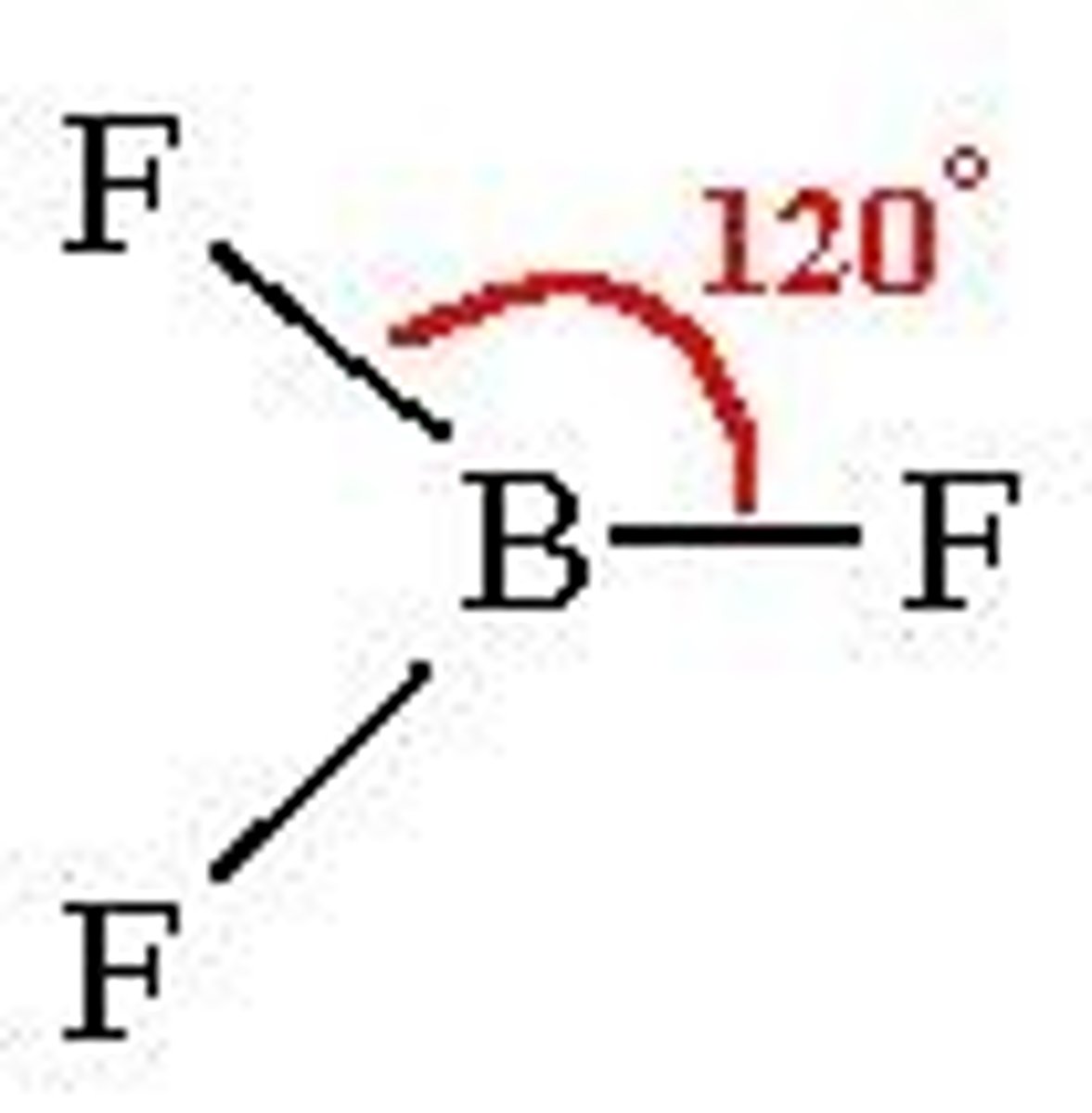
trigonal planar
-3 bonding
-0 non-bonding
-sp^2
-120 degrees
3
New cards
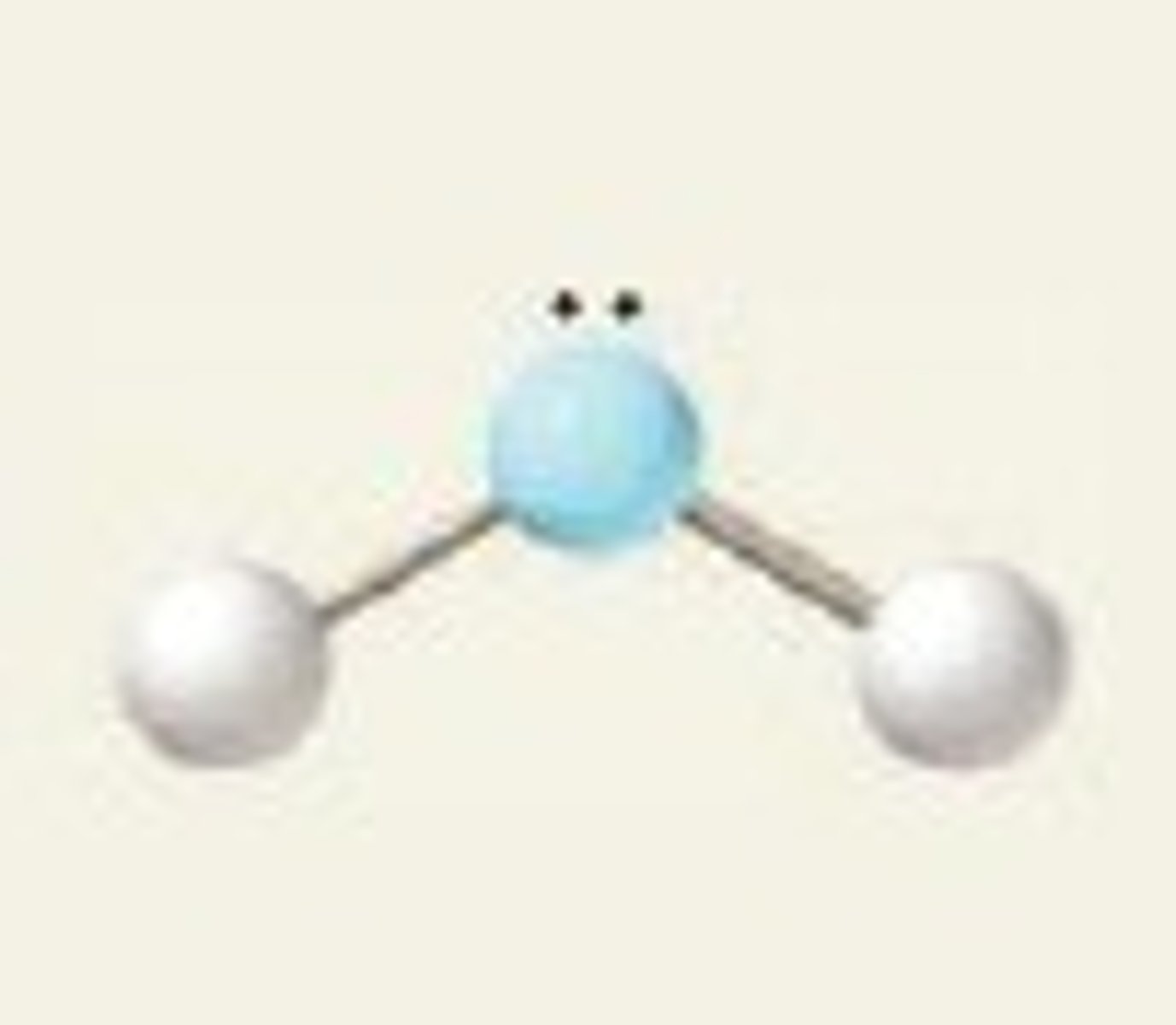
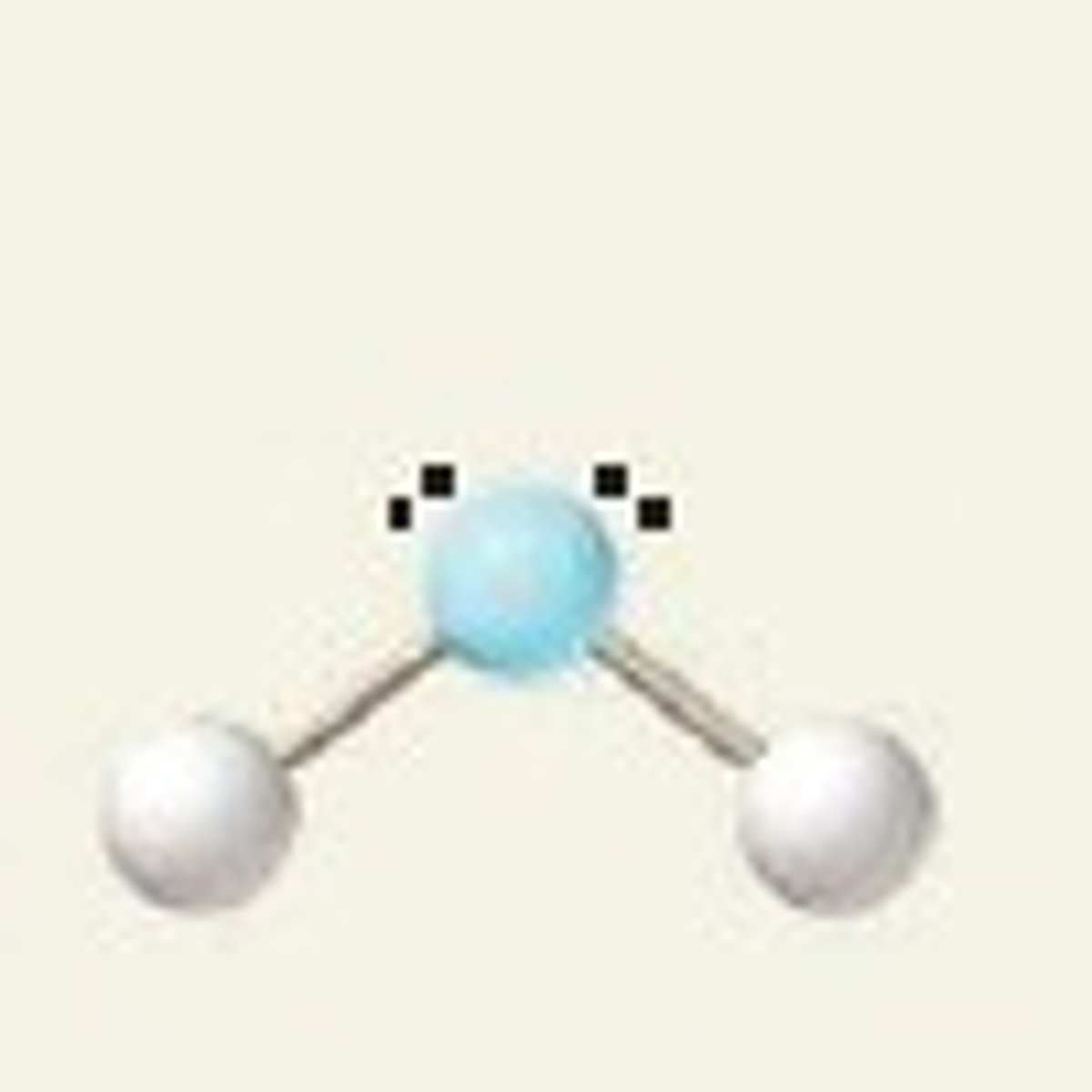
bent
-2 bonding
-1 non-bonding
-sp^2
~120 degrees
4
New cards
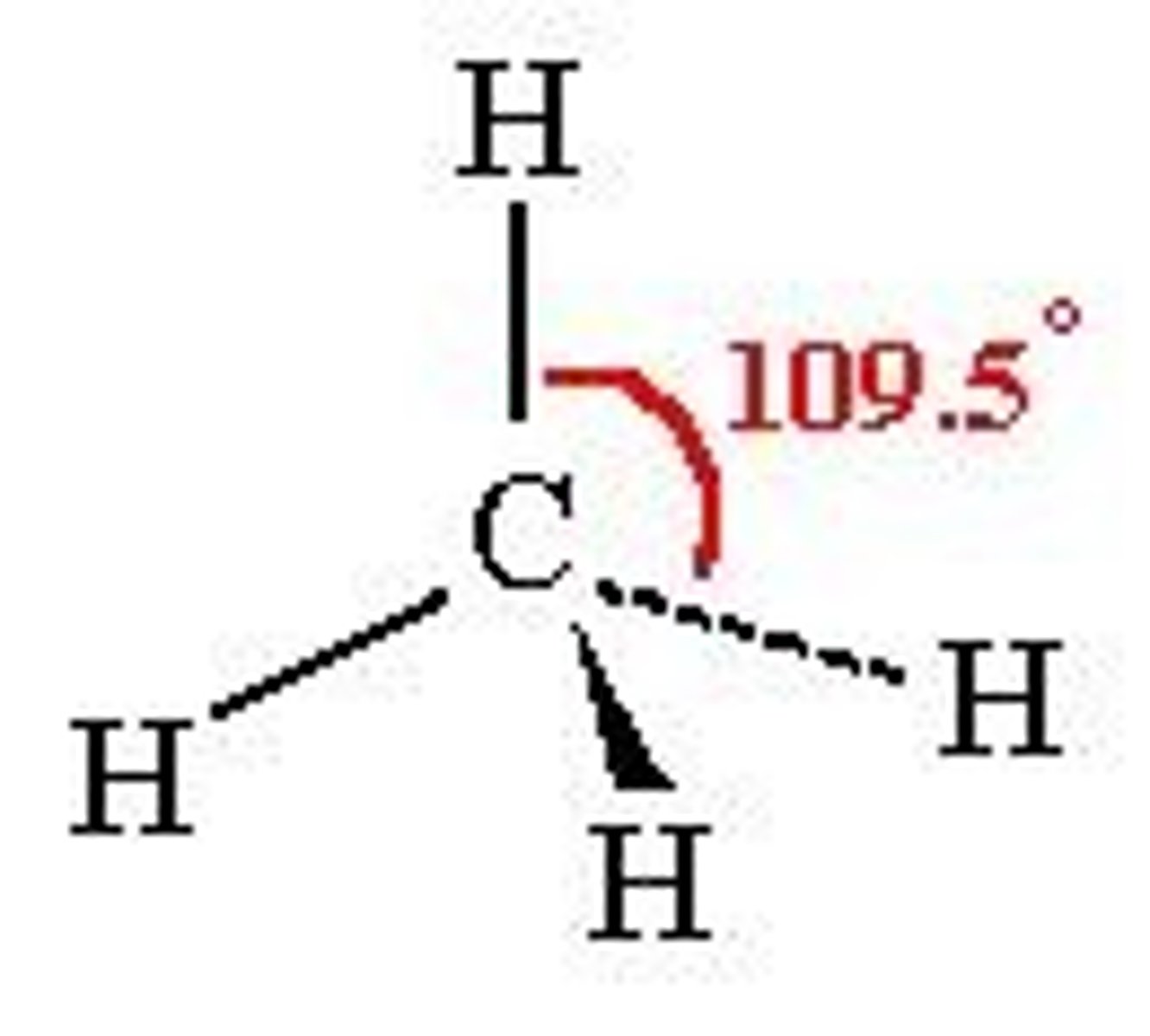
tetrahedral
-4 bonding
-0 non-bonding
-sp^3
~109 degrees
5
New cards
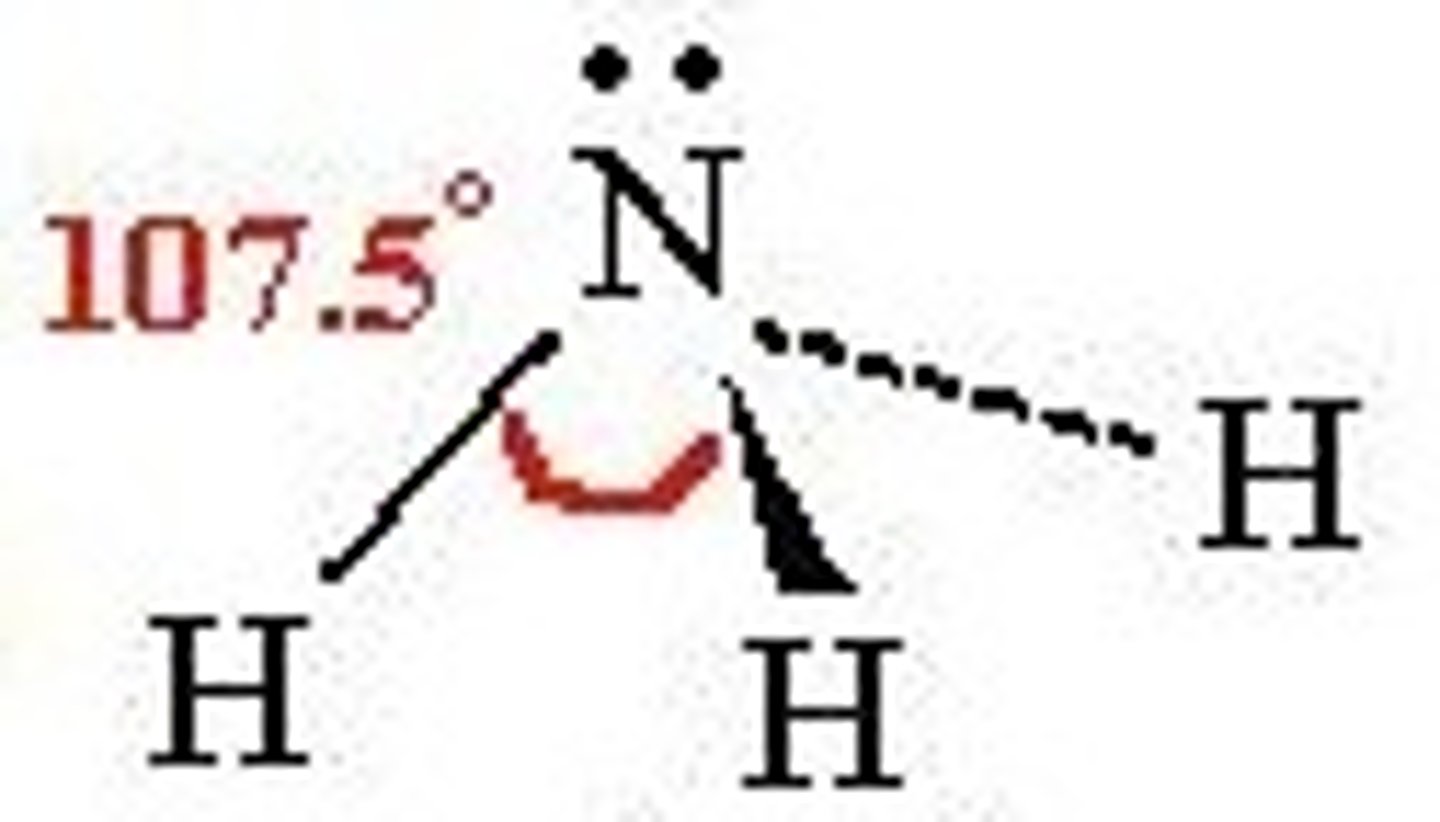
trigonal pyramidal
-3 bonding
-1 non-bonding
-sp^3
~109 degrees
6
New cards
bent pt. 2
-2 bonding
-2 non-bonding
-sp^3
~109 degrees
7
New cards