Electrolysis
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What is electrolysis?
the breaking down of an ionic compound using electricity
What is an electrode?
an electrically conducting terminal that is responsible for passing a flow of electricity into the reaction. There are 2 types of electrode:
- the anode (+) → positive terminal / electron deficient
- the cathode (-) → negative terminal / electron rich
What is an electrolyte?
a mixture that can conduct electricity
Explain why ionic compounds only conduct electricity when in molten or aqueous solution
because for something to conduct electricity, it needs both CHARGED PARTICLES that are FREE TO MOVE
- when ionic compounds are in solid form, the charged ions are in fixed positions which means that they are not free to move
- however, when ionic compounds are in molten or aqueous solution, the charged ions are free to move
Explain why covalent compounds do not conduct electricity
- because they do not have charged particles that are free to move
- this is is because their electrons are shared in a covalent bond which means that they are localised & have no overall charge
What are cations?
- positively charged ions
- they move towards the cathode because opposite charges attract
What are anions?
- negatively charged ions
- they move towards the anode because opposite charges attract
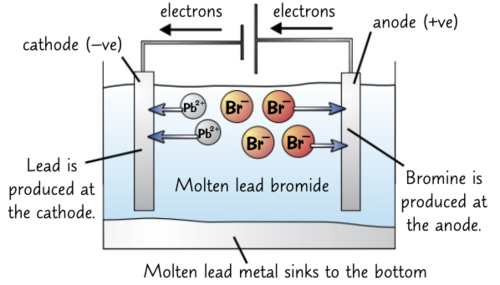
Explain what happens in the electrolysis of lead bromide (PbBr2)
Ions present: Pb2+, Br-, H+, OH-
ANODE (oxidation): 2Br- → Br2 + 2e-
CATHODE (reduction): Pb2+ + 2e- → Pb
Overall reaction: Pb2++ 2Br- → Pb + Br2
Extra notes:
- bromine gas is produced at the anode
- lead is produced at the cathode
- molten lead metal sinks to the bottom
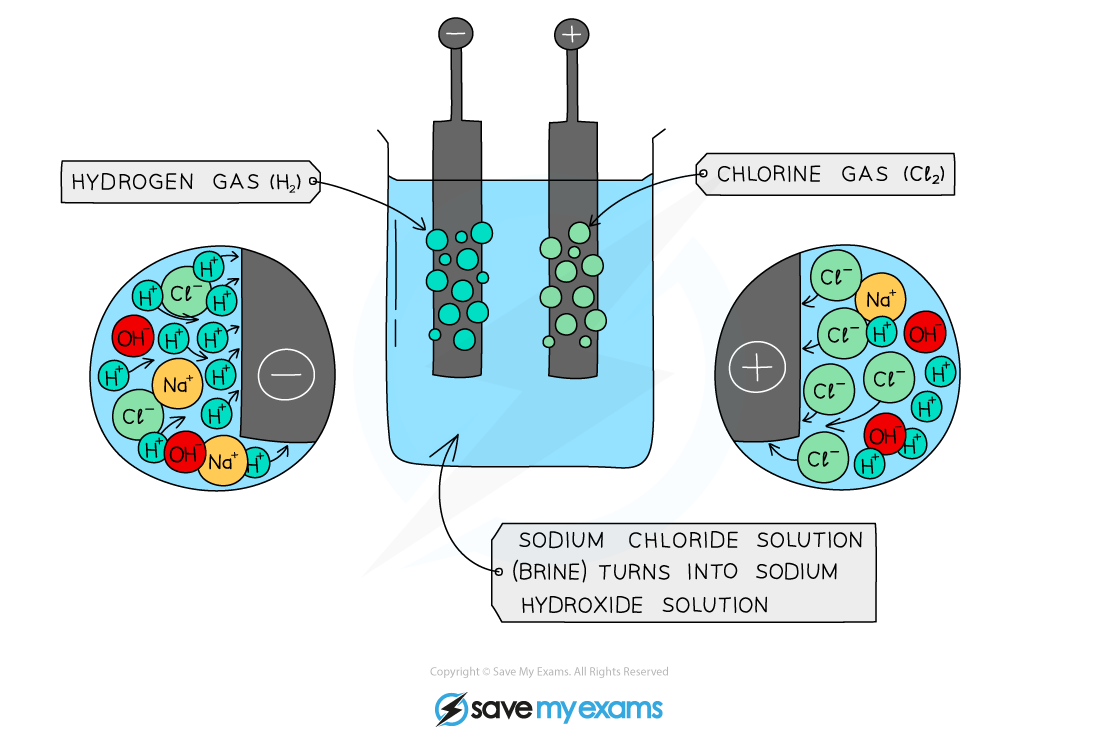
Explain what happens in the electrolysis of concentrated sodium chloride solution (NaCl(aq))
Ions present: Na+(aq) , Cl-(aq) , H+(aq) , OH-(aq)
ANODE (oxidation): 2Cl- → Cl2 + 2e-
CATHODE (reduction): 2H+ + 2e- → H2
Overall reaction: 2H+ + 2Cl- → H2 + Cl2
Extra notes:
- concentrated sodium chloride solution can also be called brine
- chlorine gas is formed at the anode
- hydrogen gas is formed at the cathode
sodium hydroxide remains in solution
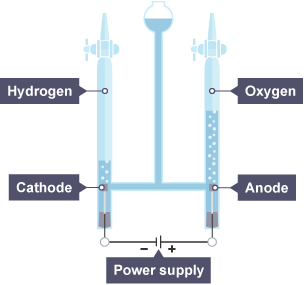
Explain what happens in the electrolysis of dilute sulfuric acid (H2SO4(aq))
Ions present: H+(aq) , OH-(aq) , SO42-(aq)
ANODE (oxidation): 4OH-(aq) → 2H₂O(l) + O₂(g) + 4e-
CATHODE (reduction): 2H+(aq) + 2e- → H₂(g)
Overall reaction: 4OH- + 4H+ → 2H₂O + O₂ + 2H₂
Extra notes:
- the ratio of hydrogen to oxygen is 2:1
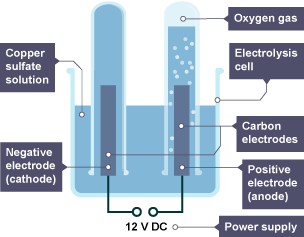
Explain what happens in the electrolysis of copper (II) sulfate (CuSO4(aq))
Ions present: Cu2+(aq) , SO42-(aq) , H+(aq) , OH-(aq)
ANODE (oxidation): 4OH- → O2 + 2H2O + 4e-
CATHODE (reduction): Cu2+ + 2e- → Cu
Overall reaction: 8OH- + Cu2+ → 2O2 + 4H2O + Cu
Extra notes:
- sulfuric acid is left in the electrolyte
What are the general rules for the electrolysis of aqueous salt solutions?
AT THE ANODE…
- oxidation occurs
- oxygen is formed unless halide ions are present which instead produces the halogen (e.g. Cl- → Cl2)
AT THE CATHODE…
- reduction occurs
- hydrogen gas is produced if the metal is more reactive than hydrogen
- pure metal is produced if the metal is less reactive than hydrogen
What does inert mean?
a substance that is not chemically reactive