6. Tissues/Adult stem cells
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
How does cellular potency change during development, and why does this necessitate the existence of adult stem cells?
Tissue development is accompanied by a progressive loss of pluripotency.
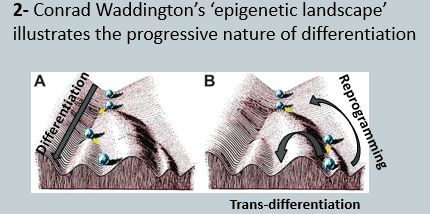
This process, conceptualized by Conrad Waddington's "epigenetic landscape," shows the progressive nature of differentiation. It sees cells "rolling down valleys" into stable, specialised fates (e.g., nerve, epithelial, muscle cells).
The aquisition of specialised function results from the expression of a subset of genes.
Fully differentiated cells have limited or no ability to divide, have restricted plasticity and limited reversability.
This means a differentiated tissue cannot easily cope with injuries or normal wear and tear on its own, creating the need for a dedicated population of adult stem cells to maintain tissue homeostasis and repair.
What are adult stem cells, and what are their key defining properties? Define cellular homeostasis in this context.
Definition: An adult stem cell (or tissue stem cell) is an undifferentiated cell found amongst differentiated cells in a tissue or organ after birth. Adult stemcellsare usually unipotent or multipotent.
They are defined by two key properties:
Self-Renewal: The ability to divide to produce more stem cells, maintaining the stem cell pool throughout an organism's life.
Uni/multipotency: Unlike embryonic stem cells, which are pluripotent, adult stem cells have limited cell fate decision and usually differentiate into one or several cell types that compose the organ. (ex: HSC can give rise to lymphocytes, macrophages, red blood cells)
Cellular Homeostasis: This is the process of maintaining a stable number of cells in a tissue by replacing old, damaged, or dying cells, or the addition of new cells as needed. Adult stem cells are essential for cellular homeostasis, which they achieve through regeneration and replacement.
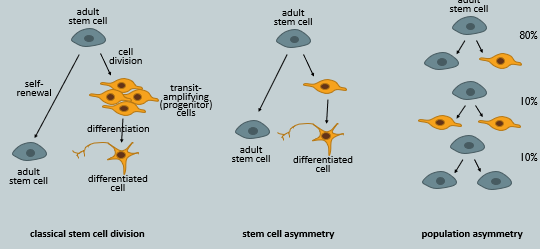
Describe the three main modes of adult stem cell division that ensure a balance between self-renewal and differentiation.
To maintain tissue homeostasis, the stem cell pool must be preserved while also producing cells for differentiation. This balance is achieved through three modes of division:
Classical Stem Cell Division (Symmetric Differentiation): A stem cell divides to produce 2 transit-amplifying progenitor cells, which then proliferate and differentiate. To maintain the stem cell pool, another stem cell must self-renew to produce two stem cells.
Stem Cell Asymmetry (Asymmetric Division): A single stem cell divides to produce one daughter cell that remains a stem cell (self-renewal) and another daughter cell that is committed to differentiation. This is the most efficient mode, simultaneously maintaining the stem cell and producing a cell for differentiation.
Population Asymmetry: This occurs at the level of the whole stem cell population. Some stem cells undergo asymmetric division (one stem cell and one progenitor) symmetric self-renewal (producing two stem cells), while others undergo symmetric differentiation (producing two progenitor cells). The balance of these events (e.g., 10% self-renewal, 10% differentiation, 80% asymmetric) maintains overall tissue homeostasis.
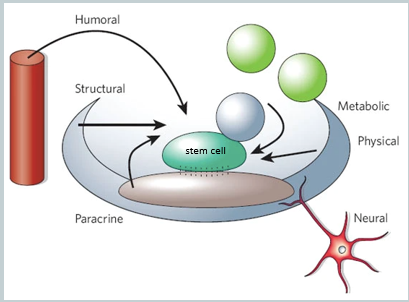
What is a stem cell niche, and what are its key components?
The stem cell niche is a specialised, tissue-specific microenvironment that regulates stem cell activity, critically maintaining the balance between self-renewal and differentiation. It provides structural support and a complex web of signals.
The components of the niche can be broadly categorised as:
Physical/Structural: Includes direct cell-cell adhesion molecules and the extracellular matrix (ECM), which anchor the stem cell and can provide regulatory cues.
Chemical/Humoral: Includes a wide range of signals such as:
Secreted proteins: Growth factors and cytokines acting over short (paracrine, juxtacrine) or long (endocrine) distances.
Neurotransmitters: Signals from nerve endings.
Metabolic factors: Local concentrations of ions like Ca2+ or molecules like reactive oxygen species (ROS).
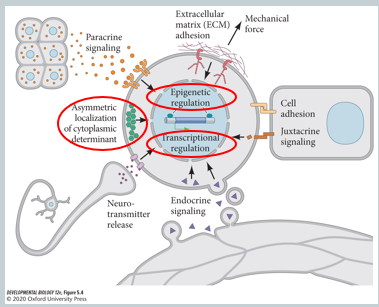
Beyond the external niche, what are the key intracellular mechanisms that regulate adult stem cell fate?
The behaviour of an adult stem cell is controlled by intrinsic mechanisms in response to niche signals. The three main categories are:
Epigenetic Regulation: Changes to the chromatin structure, such as histone modifications (e.g., acetylation, methylation) and DNA methylation, control which genes are accessible for transcription. This "epigenetic memory" influences whether a cell remains a stem cell or commits to differentiation.
Transcriptional Regulation: A network of transcription factors within the stem cell regulating genes that control quiescence (dormancy), proliferation, self-renewal, and differentiation.
Cytoplasmic Determinants: Asymmetric distribution of proteins within the stem cell's cytoplasm before it divides. When the cell splits, these determinants are inherited unequally by the daughter cells, causing them to adopt different fates (e.g., one becomes a stem cell, the other a progenitor). This is a key mechanism for asymmetric cell division.
Invertebrate adult stem cells:
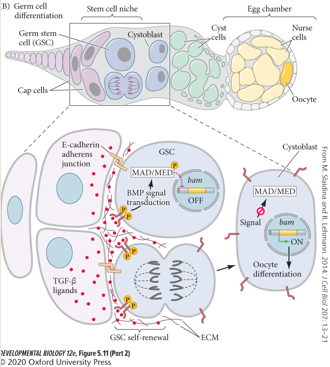
Describe the regulation of Germline Stem Cells (GSCs) in the Drosophila ovarian niche. What are the key cells, signals, and genes involved?
The Drosophila ovarian GSC niche is a classic model for stem cell regulation.
Niche Structure: GSCs are anchored to Cap cells at the anterior tip of the germarium.
Signaling Pathway: The Cap cells secrete TGFb ligands (e.g Dpp), which activate BMP signalling. BMP signalling represses bam, which is required for differentiation.
Mechanism:
1. Dpp binds to receptors on the adjacent GSC, activating BMP signaling within the GSC.
2. This intracellular signal represses the transcription of the gene bam. bam is a master regulator required for differentiation.
3. By repressing bam, the GSC is maintained in a self-renewing, undifferentiated state.Asymmetric Division: When a GSC divides, one daughter cell remains attached to the Cap cells and continues to receive the Dpp signal, thus self-renewing. The other daughter cell is displaced away from the niche, no longer receives the Dpp signal, begins to express bam, and commits to differentiation into a cystoblast, which will eventually form an egg.
ECM molecules restrict TGF𝛽 ligand diffusion.
Invertebrate adult stem cells:
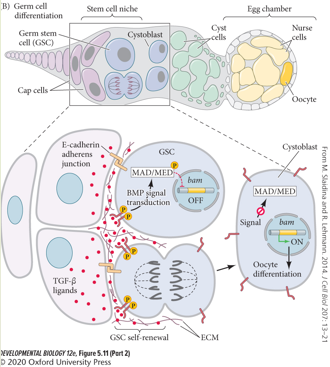
What happens if the BMP signaling pathway in the Drosophila ovarian niche is dysregulated?
The balance of BMP signaling is critical for ovarian homeostasis.
Loss of BMP signal (dpp) → GSC differentiation:
If the GSCs do not receive the Dpp signal, they can no longer repress bam. As a result, all GSCs will differentiate, and the stem cell pool will be lost. This also occurs with forced overexpression of bam.
Overexpression of BMP signal (dpp) → Germline tumours: If the Dpp signal is too strong or widespread, it represses bam in both daughter cells after division. This leads to the uncontrolled proliferation of GSCs that fail to differentiate, resulting in a germline tumour. This also occurs with loss of bam.
Vertebrate adult stem cells:
Describe the vertebrate skeletal muscle stem cell (satellite cell), its location, and its activation process.
The primary adult stem cell of skeletal muscle is the **satellite cell**.
Location: In healthy tissue, quiescent satellite cells (Pax7+) provide stem cell expansion, maintenance and commitment. They are located in a niche between the plasma membrane of the myofibre and the overlying basal lamina.
Markers: Quiescent satellite cells are defined by the expression of the transcription factor Pax7+.
Activation: Upon muscle injury, satellite cells are activated. They begin to proliferate and upregulate myogenic transcription factors like MyoD+ and Myf5+, becoming committed myogenic progenitors (myoblasts). These myoblasts then exit the cell cycle, express factors like Myogenin+, and fuse together to form new, multi-nucleated myofibres, repairing the damaged tissue. A subset of cells will self-renew to replenish the quiescent satellite cell pool.
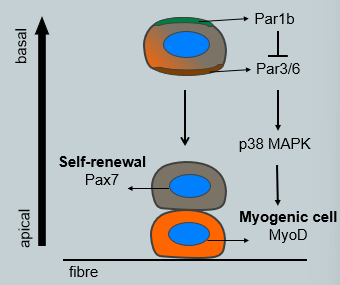
How does asymmetric cell division of satellite cells contribute to both self-renewal and muscle repair?
Satellite cells use asymmetric cell division to simultaneously produce a committed myogenic cell and renew the stem cell pool. This process is oriented by the niche.
Polarity: The satellite cell is polarized, with an apical side facing the myofibre and a basal side facing the basal lamina.
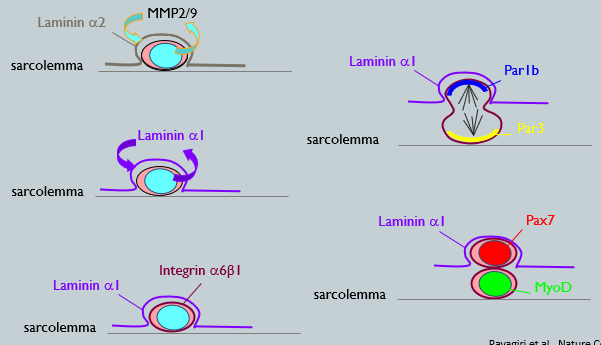
Mechanism: During division, the Par protein complex is asymmetrically segregated. Par1b localized to the basal side, where it inhibits Par3/6 on the apical side. This leads to downstream signaling (via p38 MAPK) being activated only in the basal daughter cell.
Outcome: The daughter cell that inherits the basal domain (and keeps high Pax7+ expression) undergoes self-renewal and remains a satellite stem cell. The daughter cell that inherits the apical domain activates MyoD and commits to becoming a myogenic cell destined for differentiation and fusion.
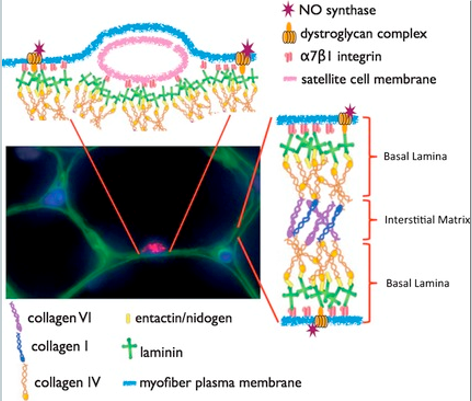
How does the satellite cell actively remodel its niche (the basal lamina) to support asymmetric division?
The satellite cell is not passive in its niche; it actively remodels its own extracellular matrix to create an asymmetric environment that reinforces its asymmetric division.
Basal lamina is a niche component, it is a Laminin-containing supramolecular structure that anchors to the cell surface (sarcolemma - plasma membrane) via the Laminin receptors.
The process involves:
Initial State: The quiescent satellite cell is surrounded by a basal lamina rich in Laminin-α2.
Activation: Upon activation, the satellite cell secretes enzymes (MMP2/9) that degrade the existing laminin.
Remodeling: The cell then deposits new laminin isoforms, creating a polarized niche. It lays down Laminin-α1 on its basal side.
Polarized Adhesion: The cell expresses Integrin-α6β1, a laminin receptor, which helps anchor it within this newly remodeled, asymmetric laminin environment.
The daughter cell that remains in contact with the laminin-rich basal lamina (Pax7+) is more likely to retain its stem cell characteristics, capable of self-renewal. Whereas, the daughter cell that moves away from the basal lamina (MyoD+) differentiates into a myoblast porgenitor.
This active remodeling of the ECM basal lamina is thought to be crucial for establishing the cell polarity (e.g. segregation of Par proteins) required for proper asymmetric division.
What are the main advantages and disadvantages of using adult stem cells for therapeutic purposes?
Advantages:
Already ‘specialised’ - induction of differentiation into specific cell types will be easier.
Plasticity - Recent evidences suggest wider than previously thought ranges of tissue types can be derived.
No Immune-rejection - if used in autologous transplantations.
No Teratomas - unlike ES cells.
No Ethical Controversy - sourced from adult tissues.
Disadvantages:
Minimal quantity - number of isolatable cells may be small.
Finite life-span - may have limited lifespan in culture.
Ageing - stem cells from aged individuals may have higher chance of genetic damage due to ageing.
Immunogenic - potential immune rejection if donor cells are derived from another individual.