Genetics lab #3 - Chi square Test and Pipettes
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
21 Terms
Chi square test
Statistical test to determine how well observed phenotypic ratios match the expected phenotypic ratios
Hypothesized pattern of inheritance supported when?
Supported if results indicate that difference between observed and expected data is due to chance
Hypothesized pattern of inheritance rejected when?
Rejected if the difference between observed data and expected data is statistically significant
Do statistics prove hypothesis
No, they support them
Null hypothesis (H0)
Difference between observed and expected is not significant and due to chance. Pattern of inheritance is supported.
Null hypothesis interpretations
“Fail to reject”: If calculated X² <= critical X² so there is no significant difference and chance is factor
“Reject”: If calculated X² > critical X² so there is significant difference
Alternative hypothesis (HA)
The difference between observed and expected data is significant. (Rejecting and accepting is opposite of null)
Degrees of freedom determination
# of phenotypes - 1 = DF
P-value and meaning
0.05
We are allowing 5% error in accepting null hypothesis
95% confident in rejecting HA and failing to reject H0
Critical Value
obtained by crossing DF and p-value
Chi square test equation and when to use
Use equation to calculate X² for each phenotype

Order of magnitude
Relative size of number referring to power of 10 that can be factored out of number
Ex: 2,500 is 2 orders of magnitude greater than 25
Sig fig rules
#1: All non zero digits are significant
#2: Zeros between nonzero digits are significant
#3: Zeros to the right of first nonzero digit are significant
#4: Zeros to the left of first nonzero digit are not significant
Molar vs Molarity
Molar (M): Concentration of solution in mol/L
Molarity: Moles of solute per liter of solvent (ex. Molarity of solution is 5 moles per liter in solution)
Equation for dilutions
C1V1=C2V2
C1 = conc. of stock solution
V1 = Volume of stock solution
C2 = conc. of final solution
V2 = volume of final solution + volume of solvent
Serial dilution
series of simple dilutions which amplifies dilution factor quickly
Key terms for serial dilutions
Aliquot: sub volume of original sample (Final volume / dilution factor)
Dilutant: material that dilutes sample (Final volume - aliquot)
Dilution factor: unit volumes in which material will be dissolved (Final conc./Initial conc. or Final vol./Aliquot)
Some rules to remember for using micropipettors
Never measure higher or lower than the range
Never turn the volume adjuster above or below range
Never let liquid into micropipettor
Always use appropriate tip
Never invert or lay down when liquid is in tip
Never let plunger snap back when filling or ejecting liquid
Never immerse barrel in fluid
Micropipettor readings
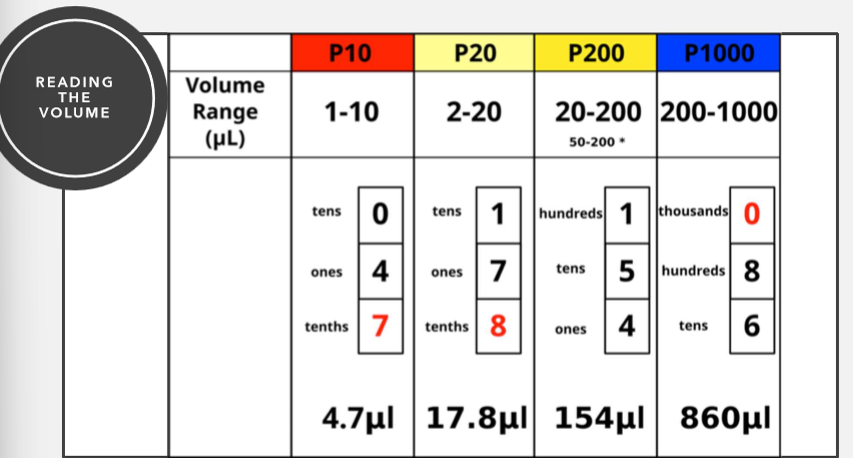
How to take up sample with micropipetter
Hold micropipetter and tube at about eye level
Press plunger down to first stop then put tip in fluid
slowly release plunger to get fluid into tip
How to expel fluid with micropipetter
Hold micropipetter and tube at eye level
Touch micropipetter to tube wall to create surface tension that helps to get fluid out of tip
slowly press plunger down to second stop to expel fluid
Hold plunger down and remove from fluid to ensure none is taken back up
After removing dispose of contaminated tip