Crystal Field Theory
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
how to work out the dn configuration of a metal ion in a complex
Subtract the oxidation state of the metal from the group number
What is crystal field theory
A purely ionic description of bonding in TM complexes.
It predicts the splitting of d-orbitals in complexes of known geometry.
It can be used to predict spectroscopic and magnetic properties in TM complexes.
What assumptions does crystal field theory make
The metal centre and ligands are point charges
Bonding arises solely via electrostatic interactions between these charges
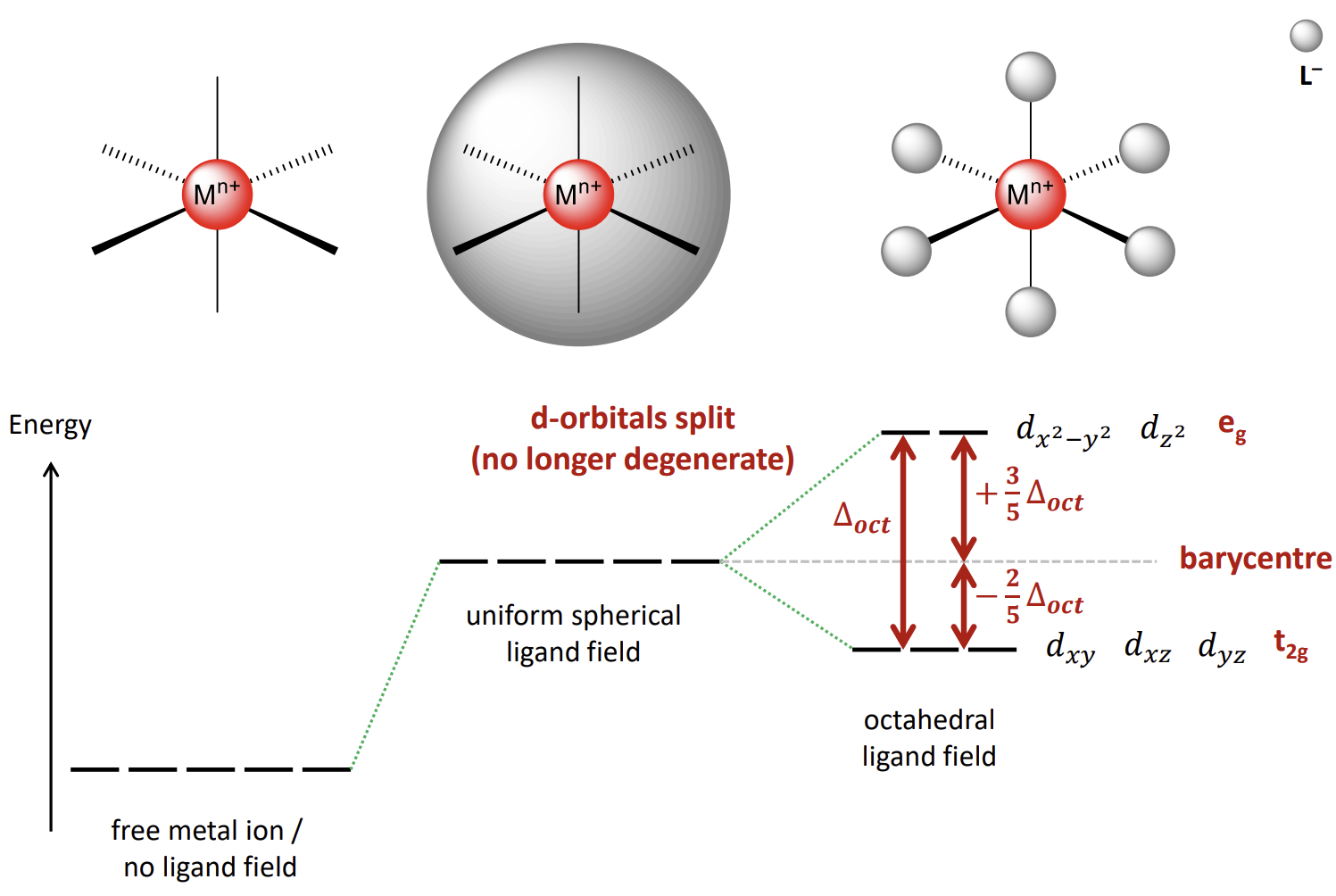
What results in the splitting of energy of the d-orbitals
We consider the ligands as negative point charges - hence they repel the electrons in the metal d-orbitals.
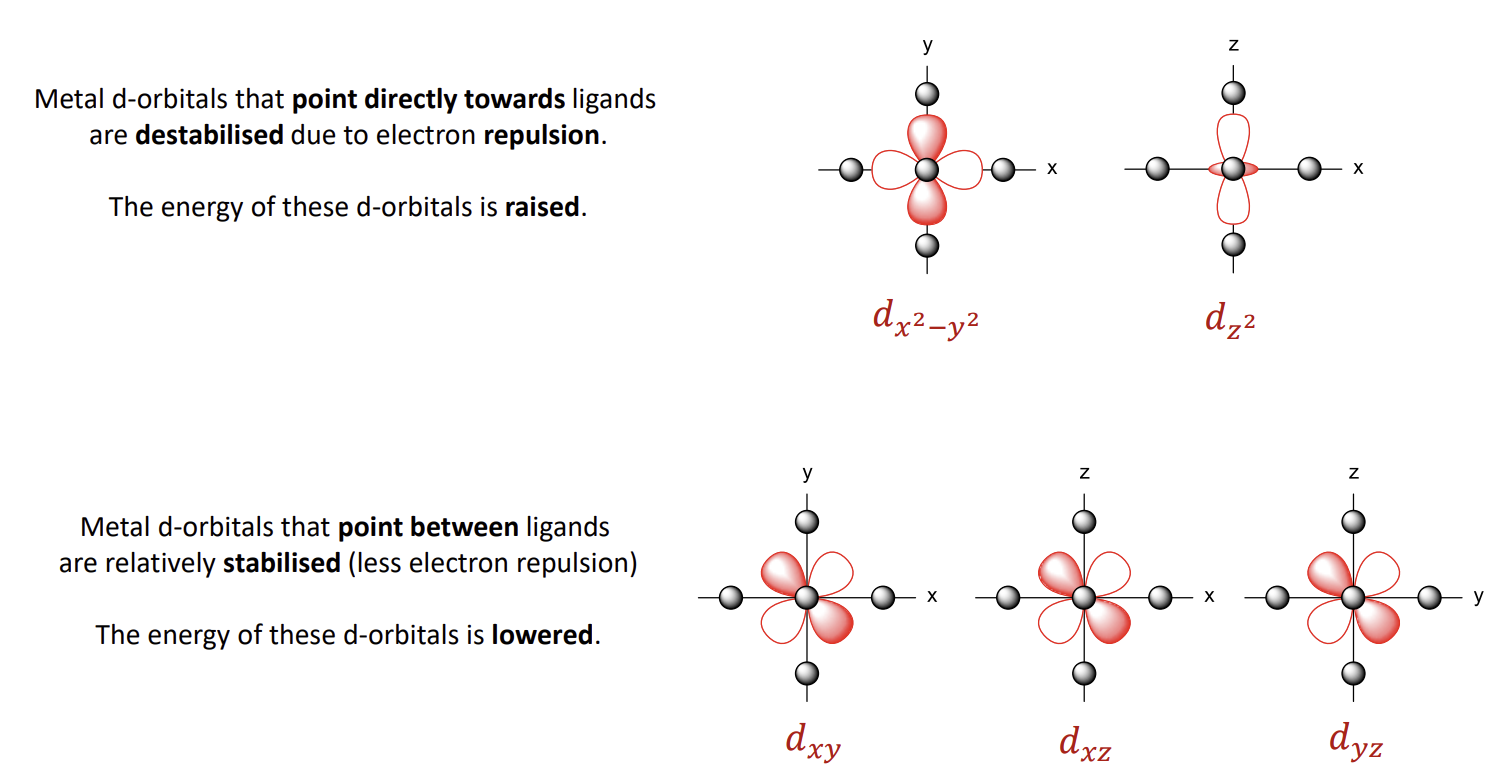
What are the eg and t2g orbitals
eg - 2 destabilised orbitals that are higher in energy. e stands for equal and g is a symmetry label (gerade)
t2g - 3 stabilised lower energy orbitals
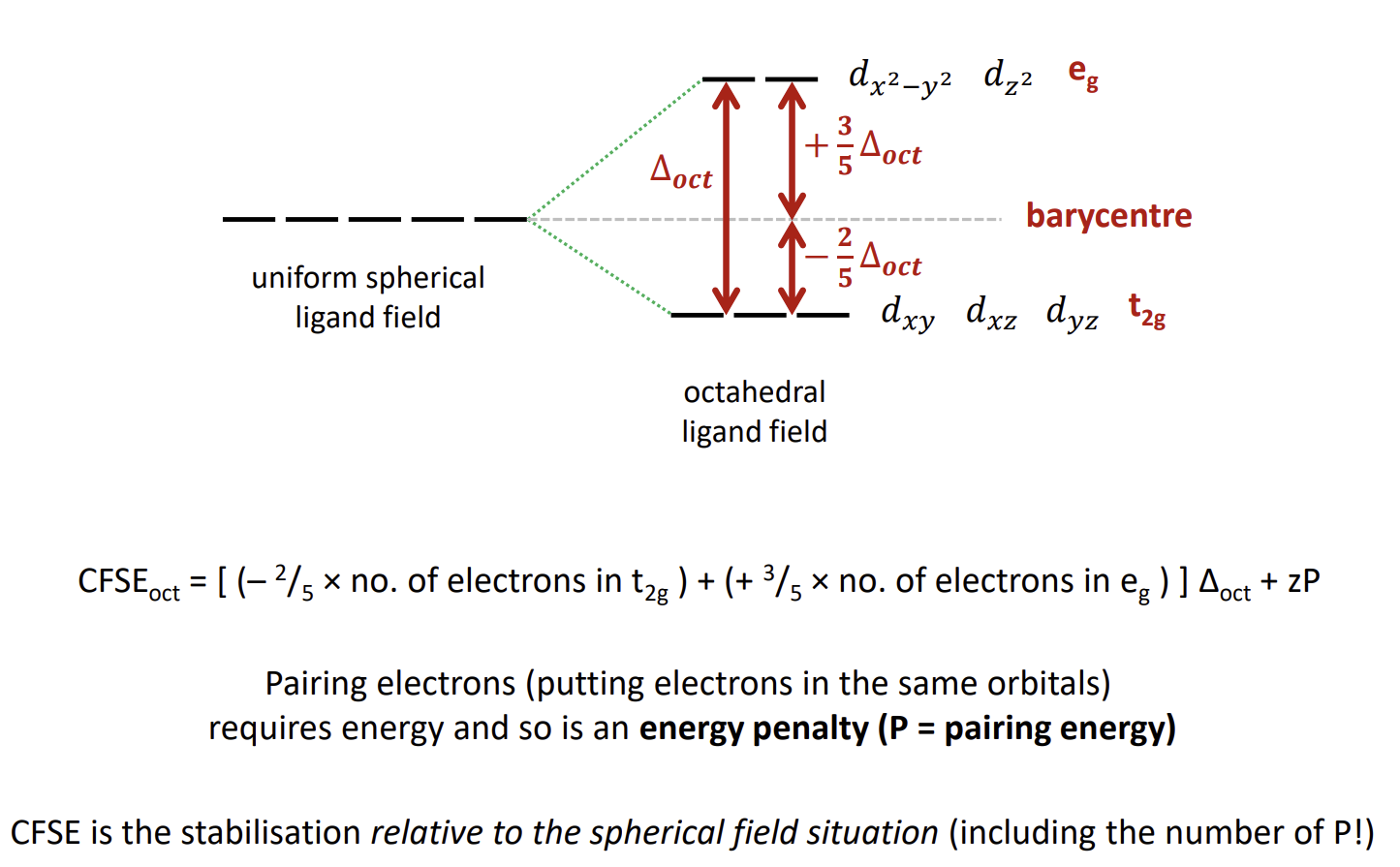
What do the orbital energy levels look like
The barycentre is the energy of the orbitals if we consider the ligands to be a sphere of negative charge that equally destabilises each d-orbital.

What is crystal field stabilisation energy
A measure of how much more stable a complex is thanks to the splitting of the d-orbitals in a particular arrangement.
what is ∆oct
The Crystal Field Splitting Parameter for an octahedral complex. It is the energy gap between the stabilised and destabilised orbitals.
The average energy of the orbitals is the barycentre energy
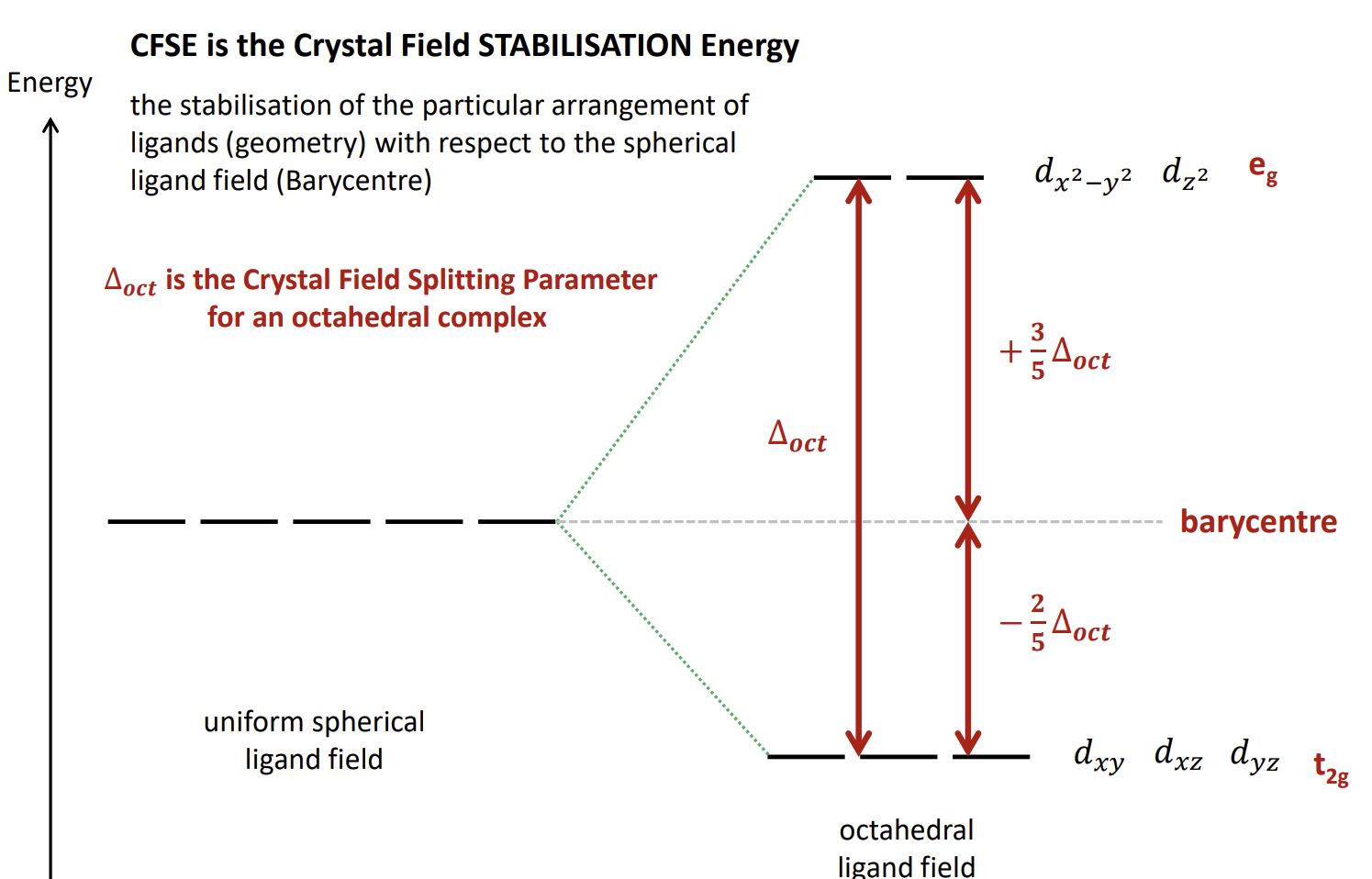
Overall diagram of crystal field theory
How do you calculate CFSEoct (stabilisation energy)
What determines the colour of a complex
The energy gap between the split d-orbital energies (wavelength of absorbed light)
What can happen when we add a 4th d-electron
The electron can sometimes go in the higher energy orbital rather than pairing up if the energy gap is small enough that the repulsion pairing energy exceeds it.
This gives rise to high and low spin configurations
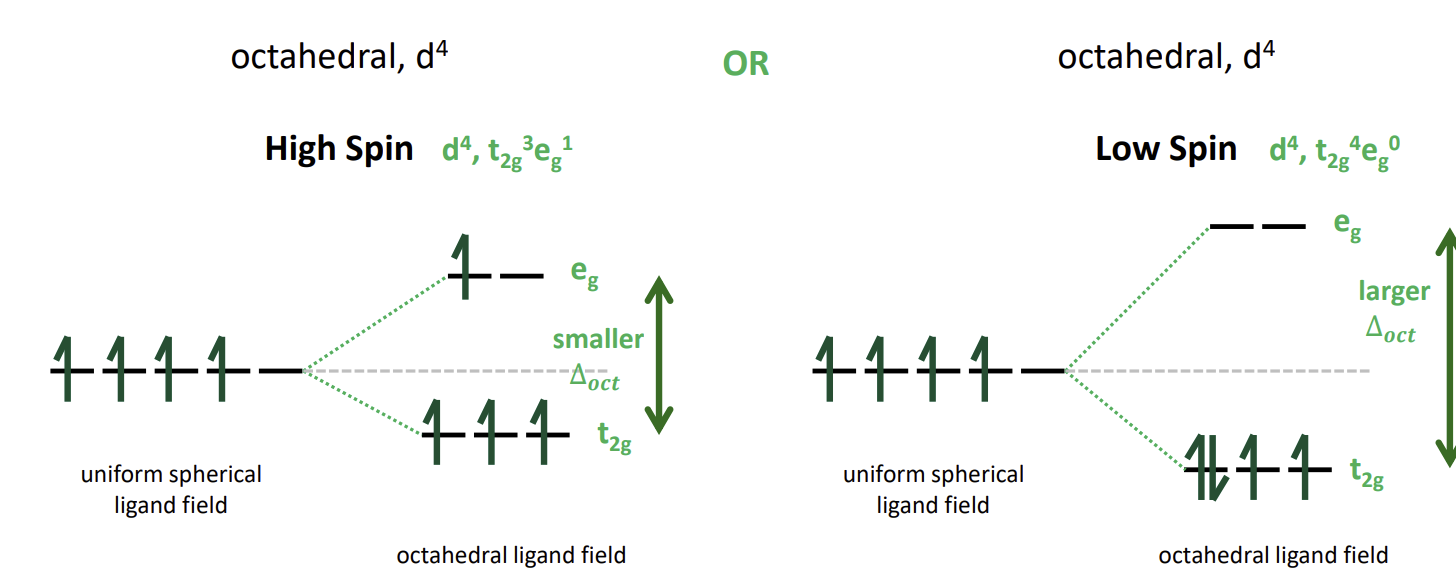
High vs low spin
High spin complexes form if ∆oct < P (pairing energy)
So ∆oct and P are the factors affecting whether a complex is high spin or low spin.
P is dependent on the metal and its oxidation state and ∆oct is additionally affected by the ligands.
How do you calculate CFSE for d4+ compounds
Same as normal but you need to add the pairing energy for each pair of electrons.
For d6+ don’t forget the pairing energy present in the spherical ligand field at the start as CFSE relies on the difference between the energies
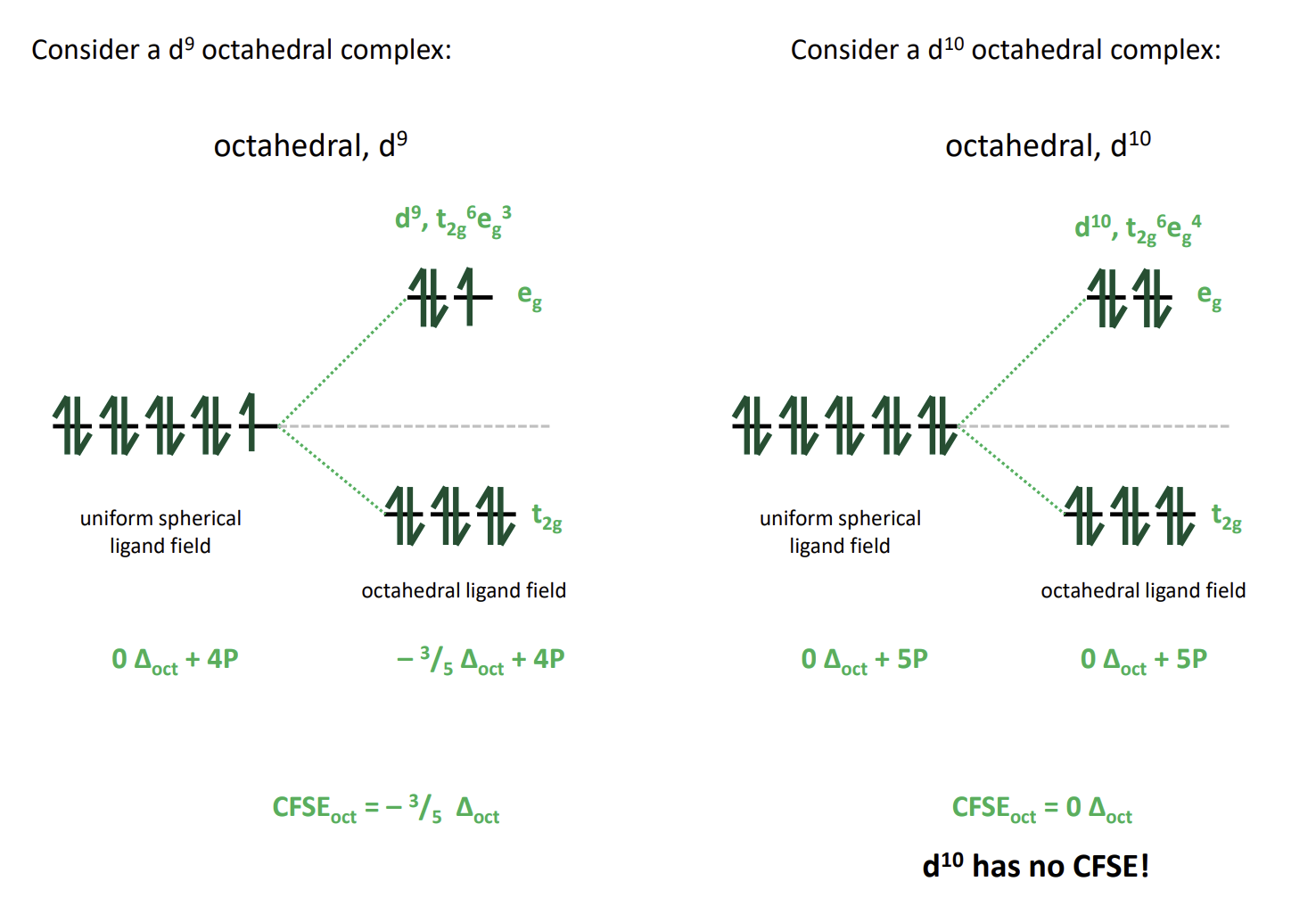
What is different about d8-10
They have no high or low spin states as the configuration ends up the same either way as electrons will be paired in the lower energy orbitals first every time.
d10 actually has no CFSE as the stabilisations and destabilisations cancel out as the orbital energies must cancel out
How does ∆ change with oxidation state of the metal
Increases with increasing oxidation state of the metal as the ligands are pulled closer so there is a larger repulsion between electrons in d-orbitals and ligands, increasing the d-orbital splitting.
How does going down the group affect ∆oct
It increases as d-orbitals get bigger so interact more with the ligands so more destabilising.
Also, the pairing energy decreases as there is more room to pair electrons.
Hence, all of the second and third row octahedral complexes are low spin
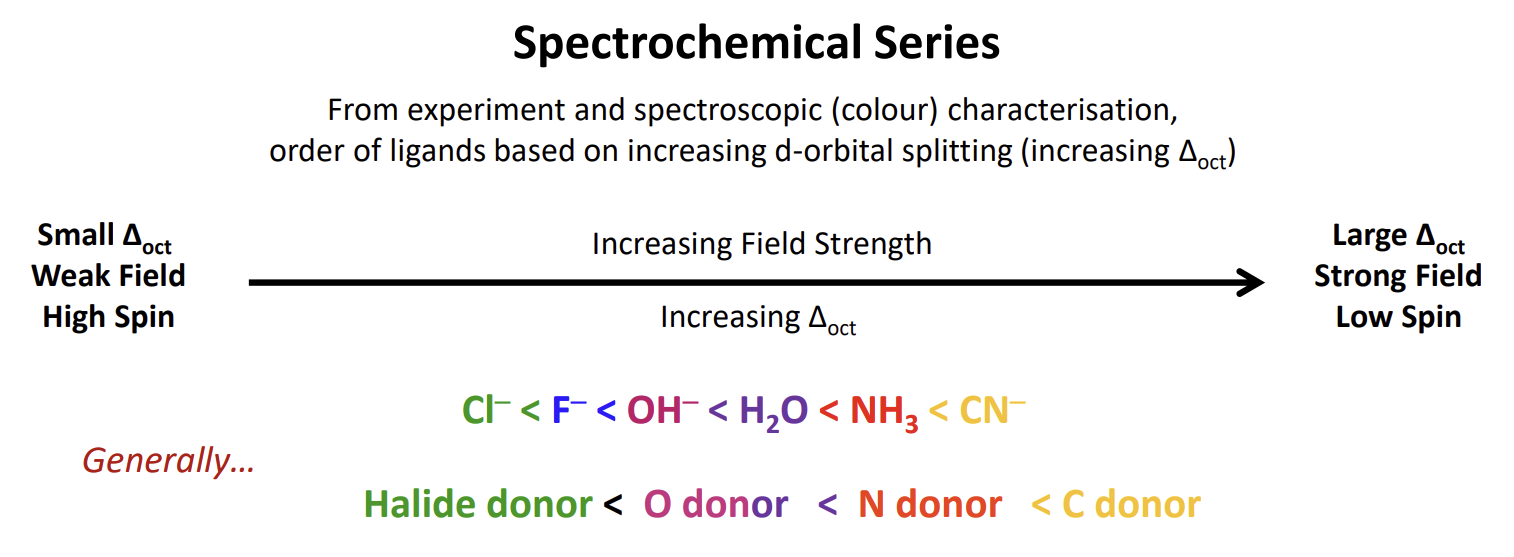
What determines ligand field strength
Ordered by periodic table based on electronegativity. More electronegative ligands hang onto their electrons more strongly and negative ligands are better sigma donors as they want to donate away the extra electrons.
What is the determinant of ligand field strength
How good a pi acceptor they are. This generally follows sigma donation but not perfectly
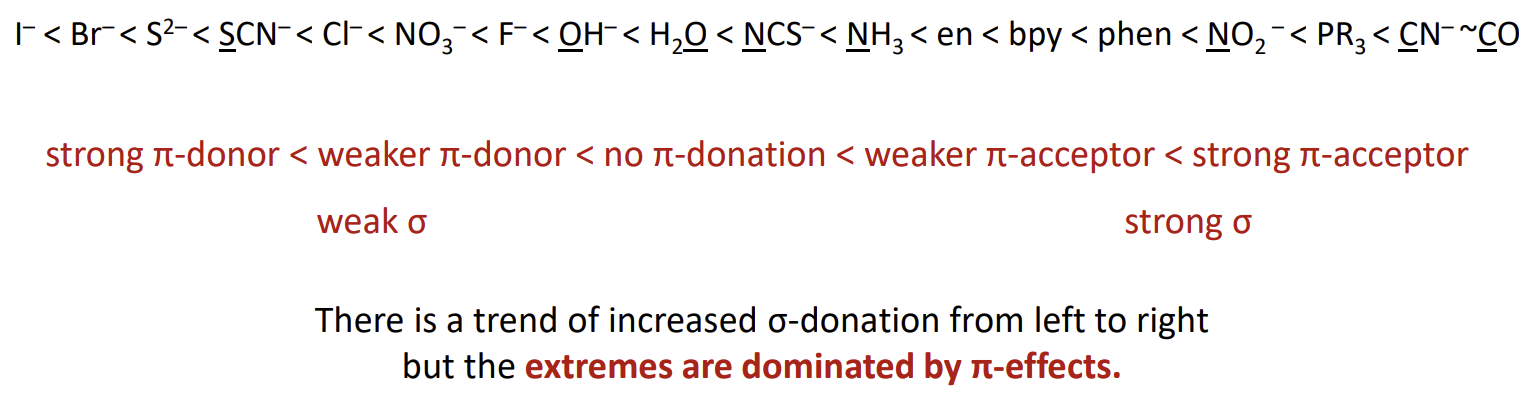
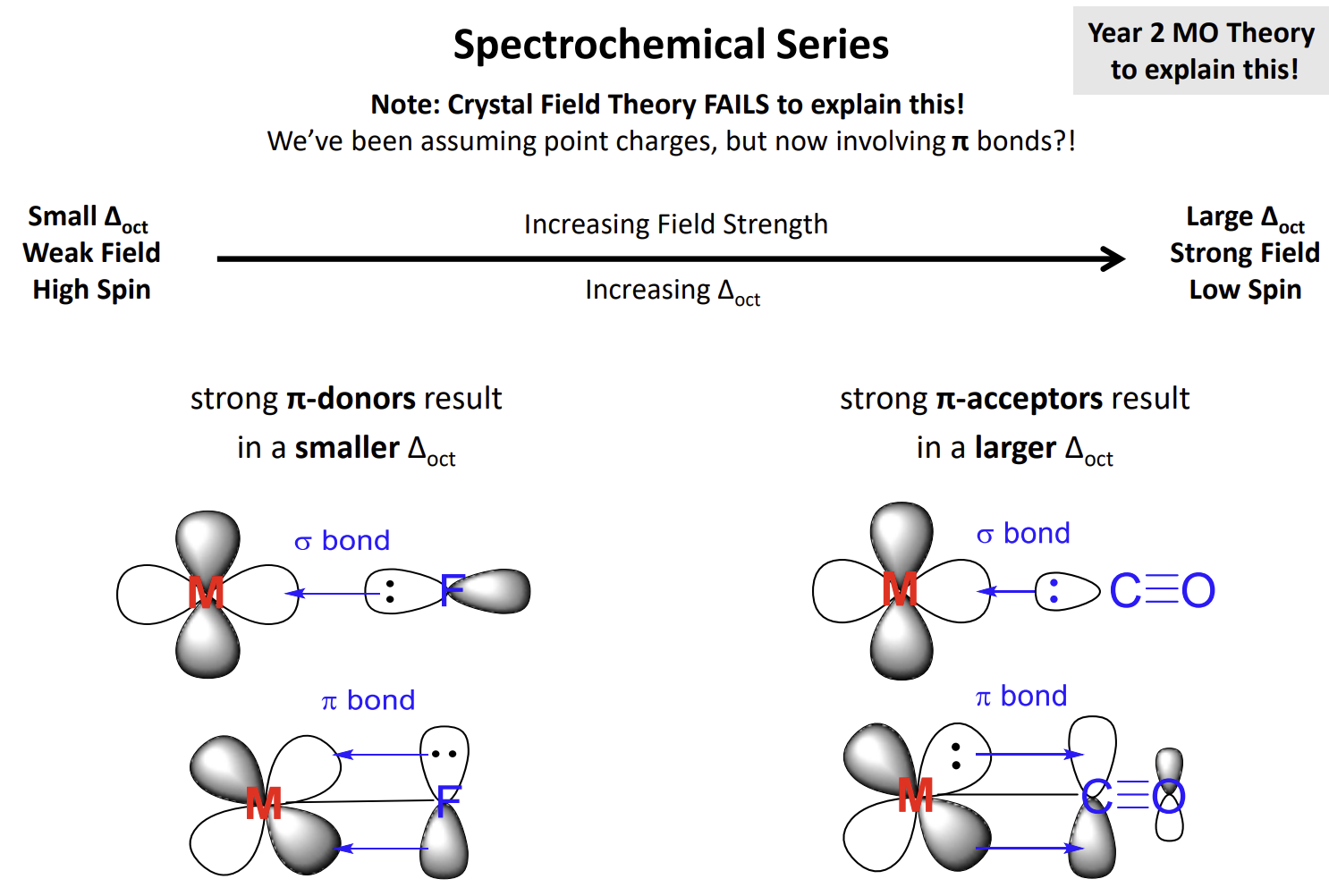
What is the problem with this determinant
Crystal Field theory cannot explain this as we assume point charges hence there are no covalent interactions
F- is a pi donor due to the presence of its lone pairs while CN- is a pi acceptor due to the presence of a pi bond in the ligand resulting in an empty pi* orbital being present which can accept a lone pair from the metal centre.
Why is I- a weaker field ligand than F-
Because its lone pairs are in larger orbitals it can donate them more easily hence it is a stronger pi donor
Why is P typically a stronger field ligand
It has low-lying d-orbitals hence it is a pi acceptor
How do things look for tetrahedral ligand fields
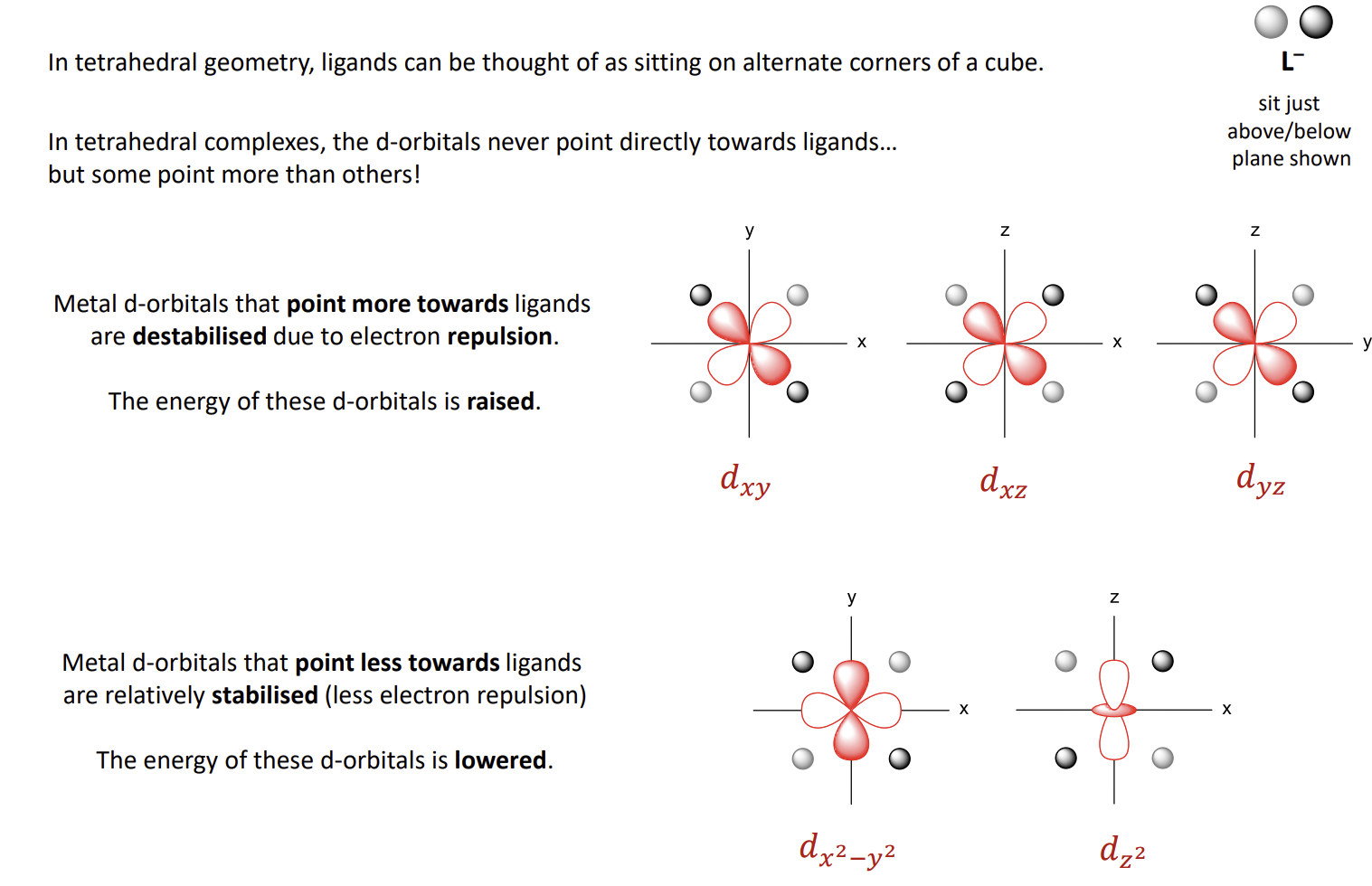
How does the energy diagram look for tetrahedral ligand fields
note that the symmetry labels are gone and e and t2 have swapped
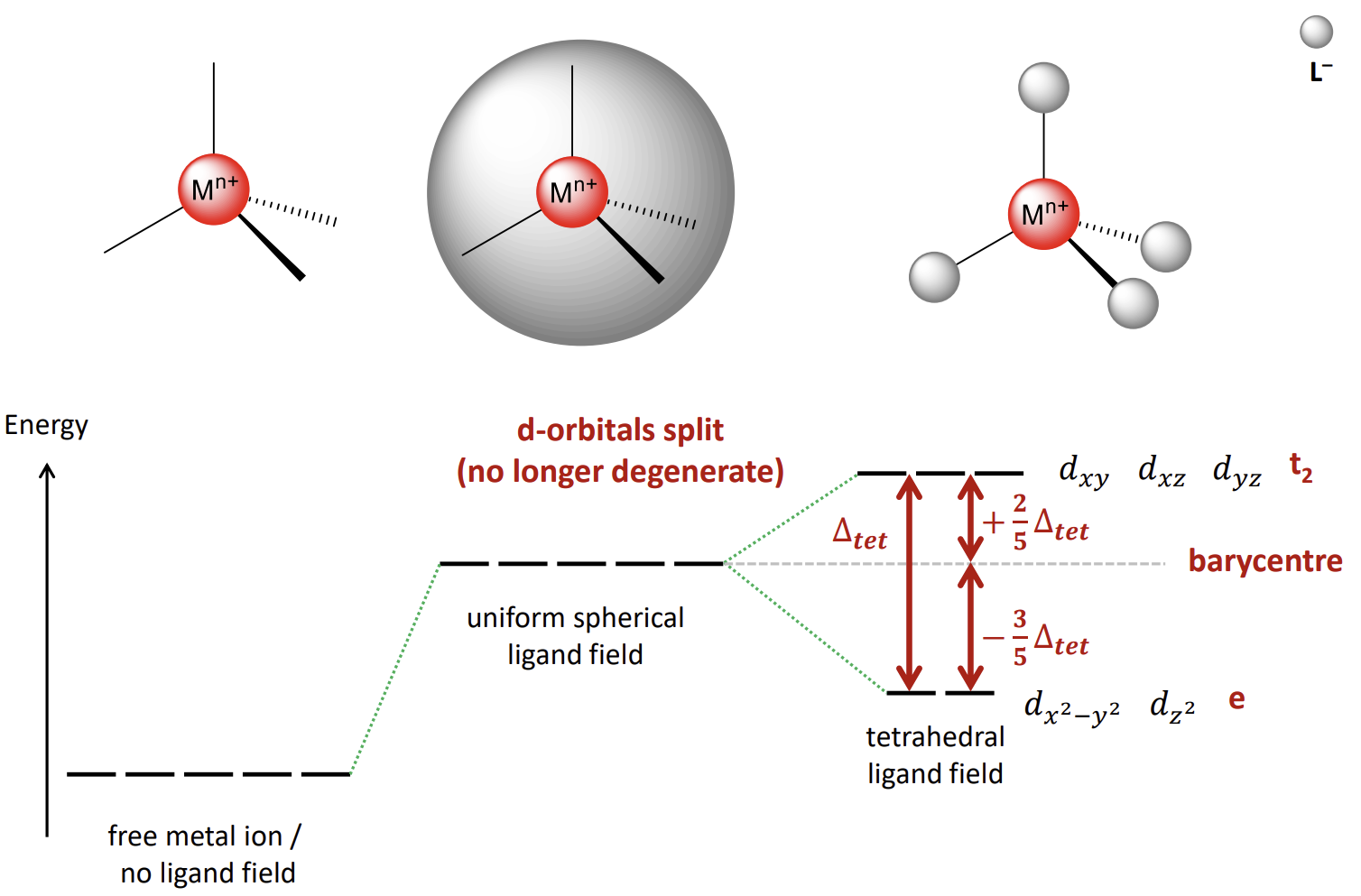
How do you calculate CFSEtet
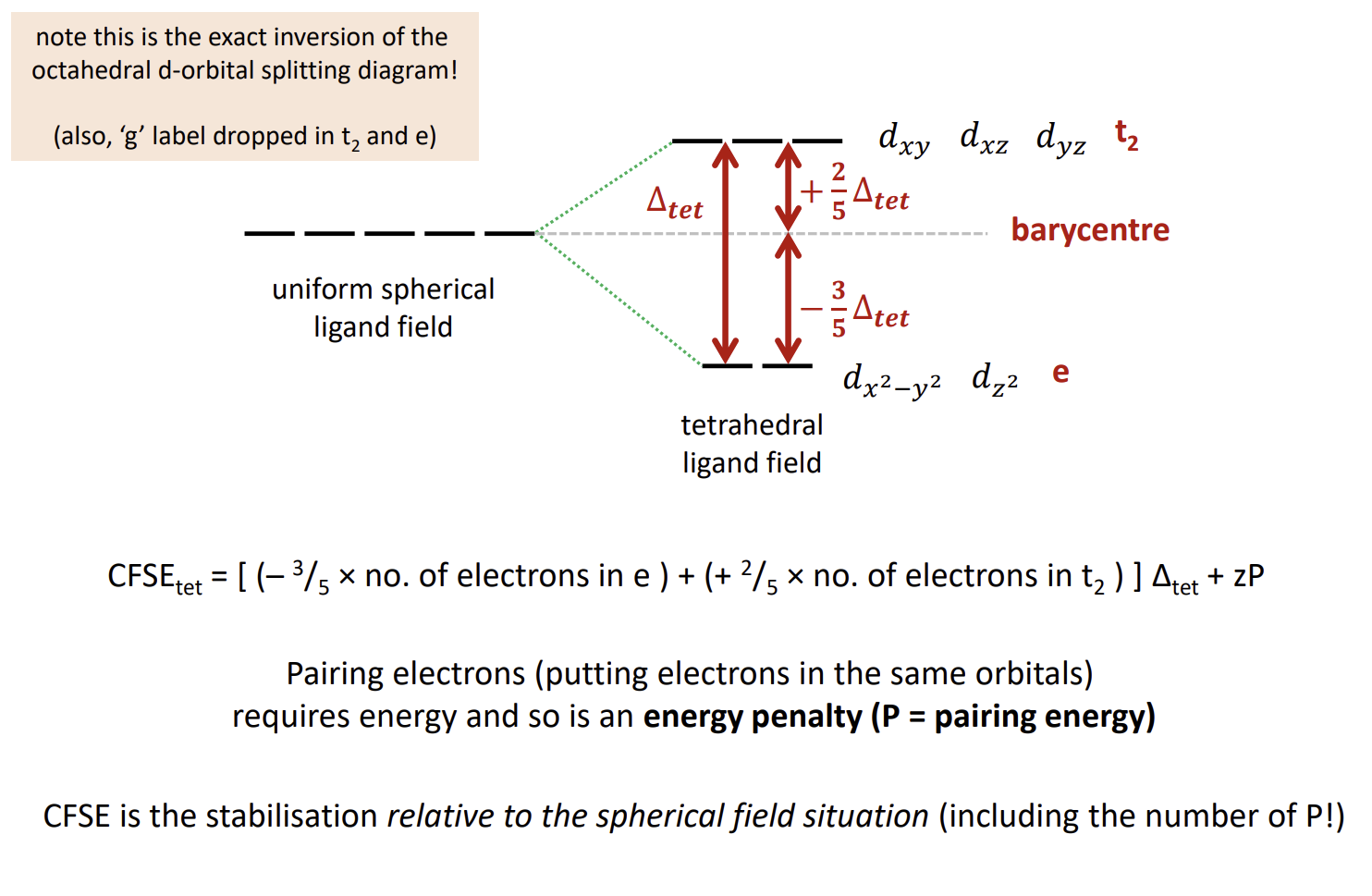
Differences between octahedral and tetrahedral ligand fields
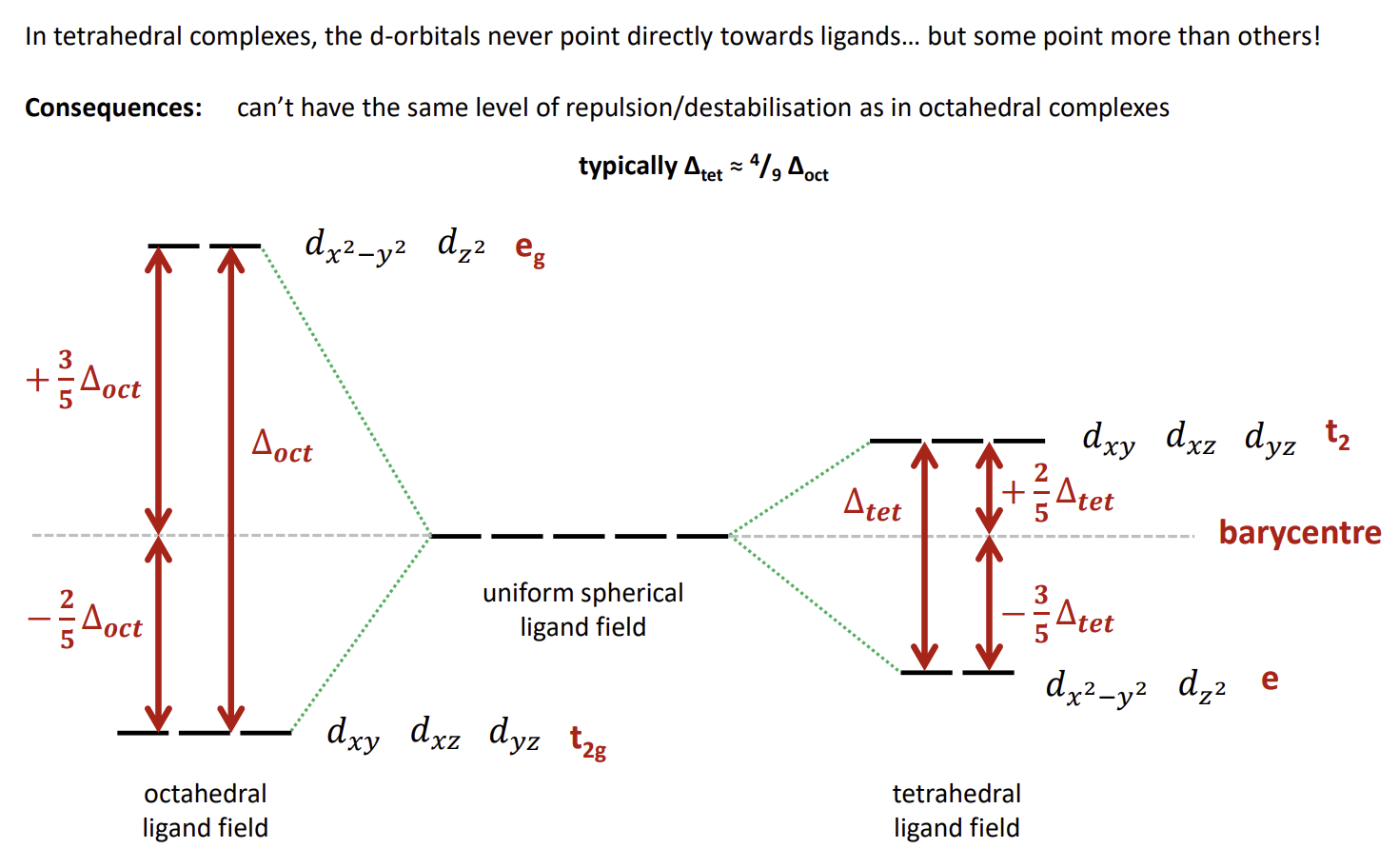
What is a consequence of ∆tet values being smaller than ∆oct
Tetrahedral complexes are always high spin as pairing an electron is not worth the energy penalty compared to promoting an electron
How can we easily work out the square planar orbital energy diagram
Remove the axial ligands from an octahedral complex
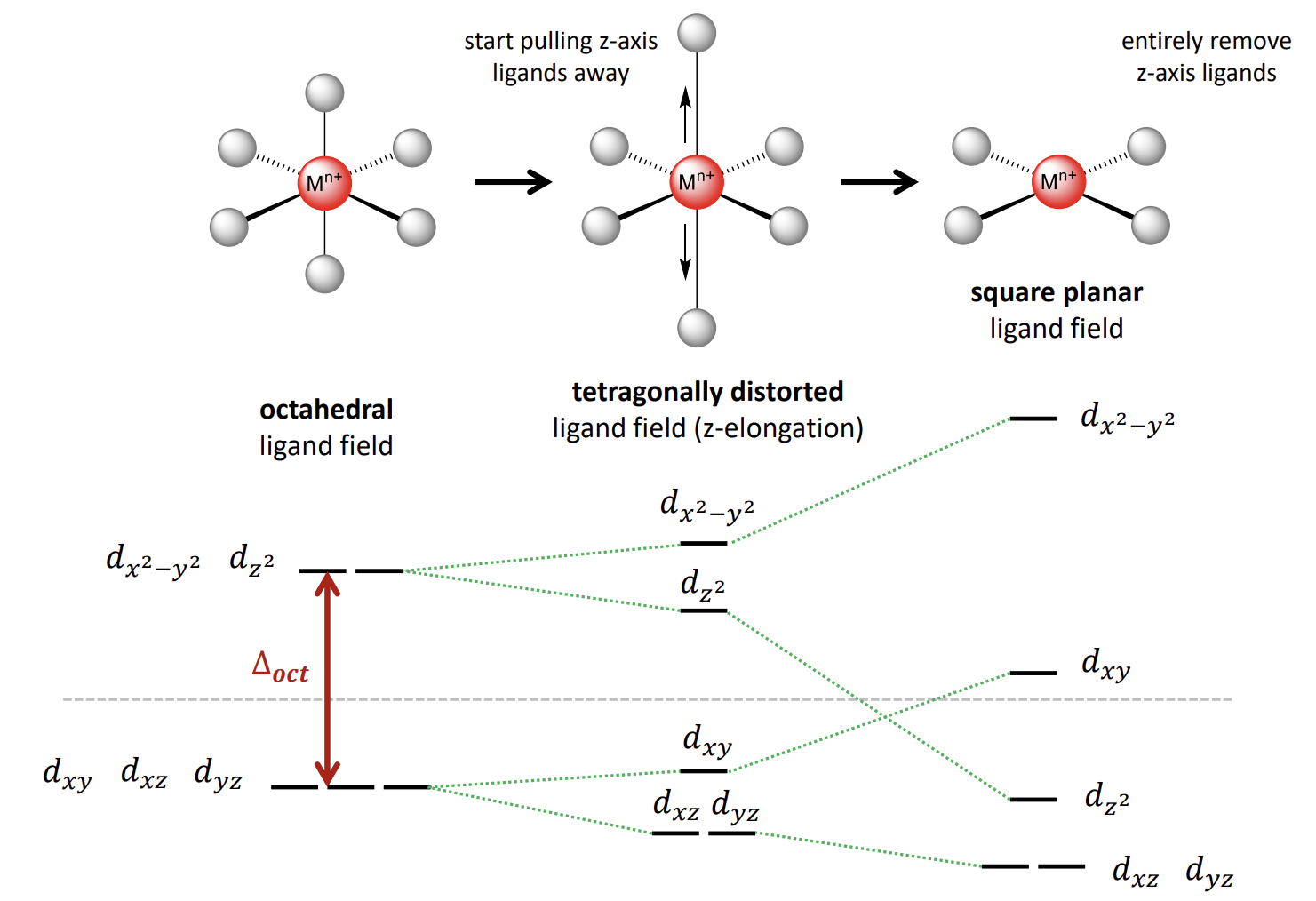
When are complexes tetrahedral or square planar
Tend to be tetrahedral as it is better for sterics
But square planar is electronically very stable for d8 complexes. The complex will be square planar if ∆ is large:
3d8 with strong field ligands (strong field gives large ∆)
4/5d8 with any ligand (larger d-orbitals give larger ∆)
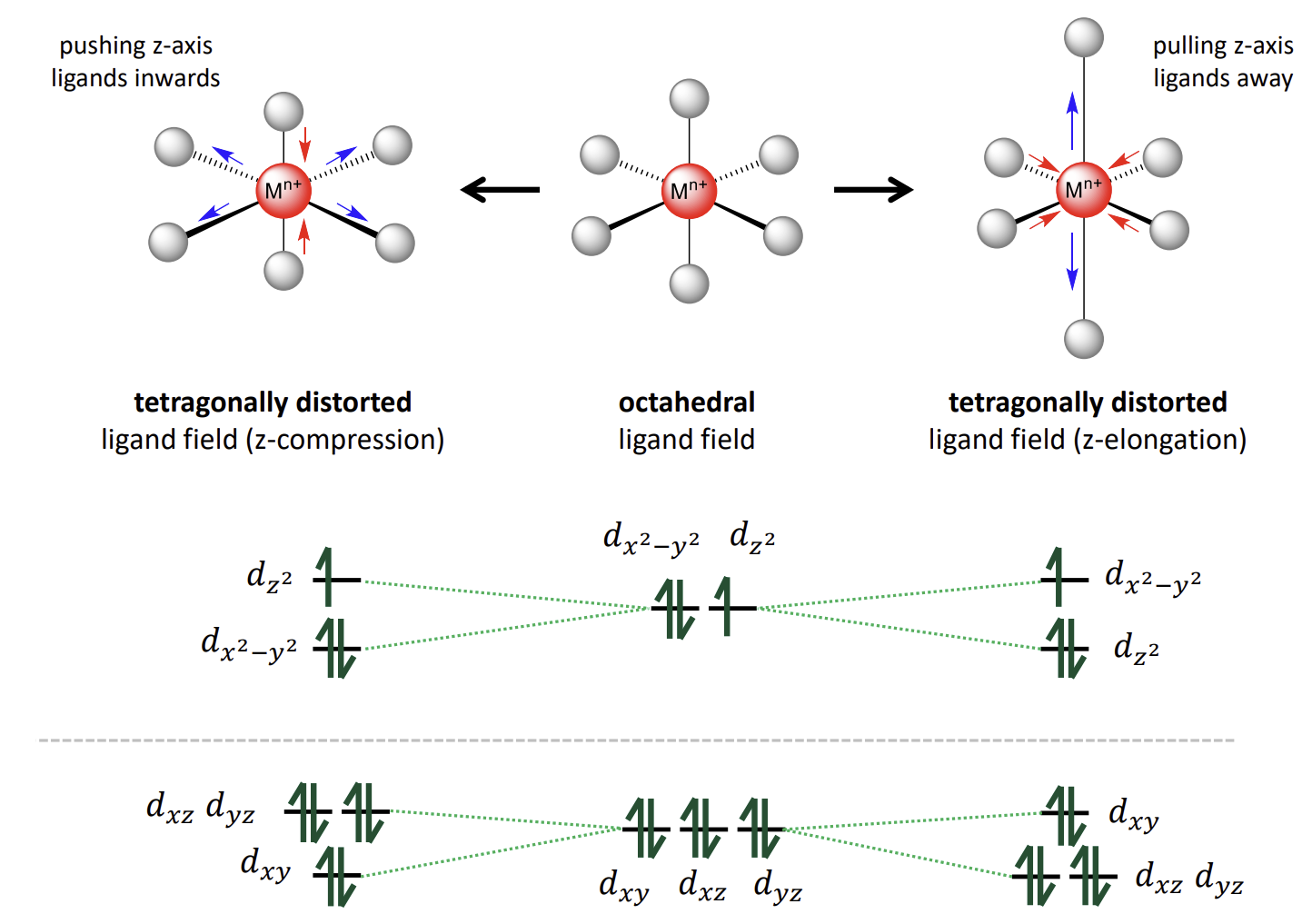
what happens in d9, high spin d4 and low spin d7 complexes
Degeneracy leads to being able to place the paired electron in one of two orbitals of equal energy
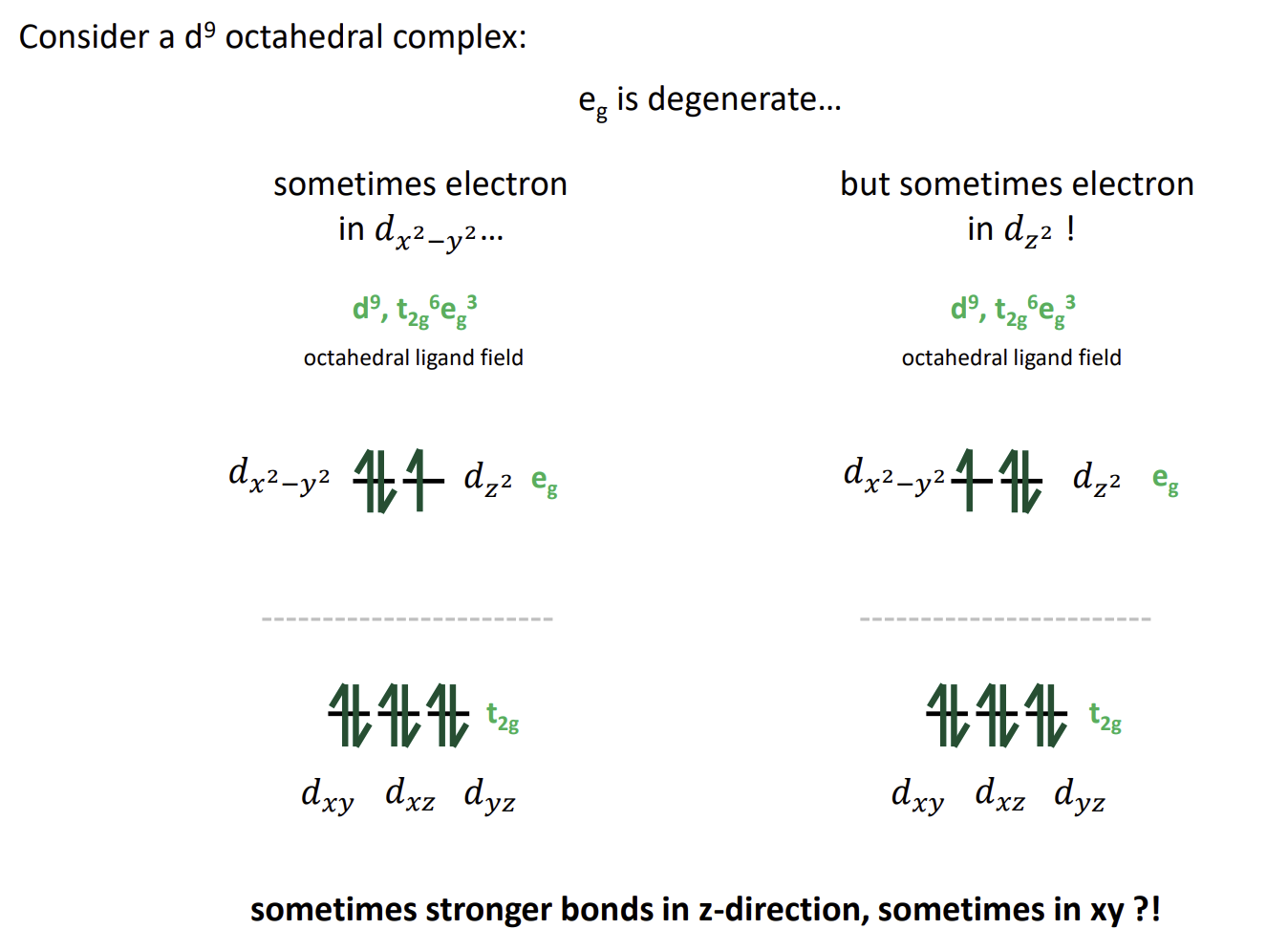
Jahn-Teller effect
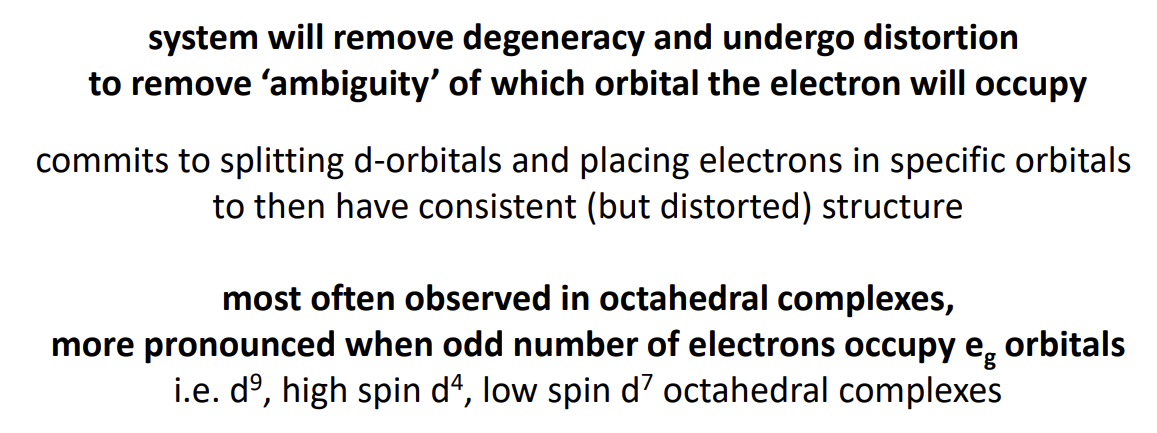
How does the complex distort