Biological Molecules
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
82 Terms
organic molecules
any molecule containing carbon that can be found in living things. The 4 classes are:
carbohydrates: respiratory substrates that provide energy for cells, used for structure in cell membranes and cell walls
proteins: main component of many cellular structures, form enzymes and chemical messengers e.g RNA
lipids: can be used as respiratory substrates to provide energy for cells, from a bilayer in cell membranes and make up some hormones
nucleic acids: form polymers(DNA and RNA) to makeup genomes.They code for the sequence of amino acids to makeup all proteins
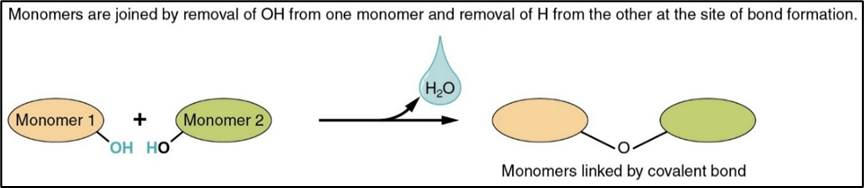
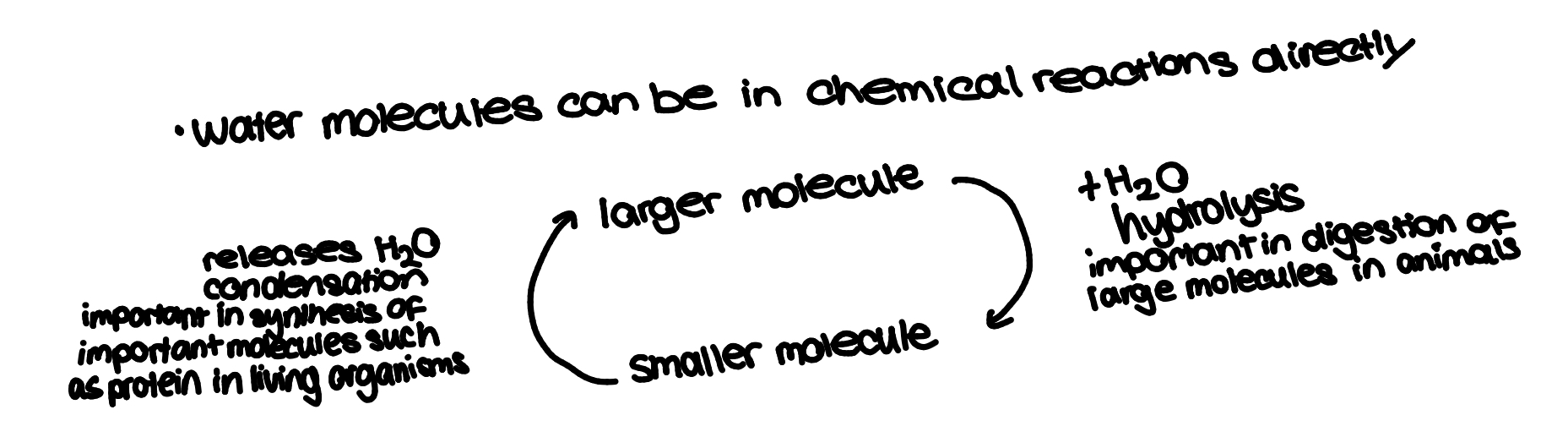
hydrolysis
breaking down of large molecules into smaller ones by addition of water molecules(like the opposite of condensation)
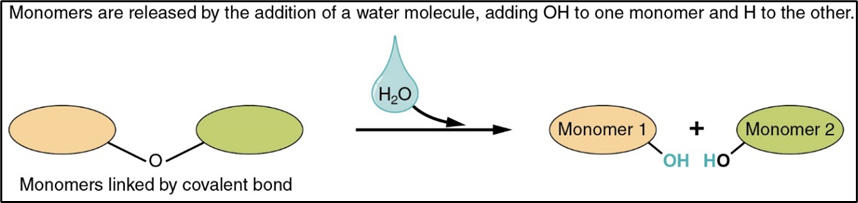
condensation reaction
chemical process when 2 molecules combine to form a more complex one whilst eliminating a simple substance(usually water)
how many biological polymers are formed
what supports the theory that all organisms share a common ancestor
all organisms use the same nucleic acid(DNA and RNA) as genetic material
all build proteins using the same 20 amino acids
all use lipids and carbohydrates as energy stores and to make up their cell membranes and walls
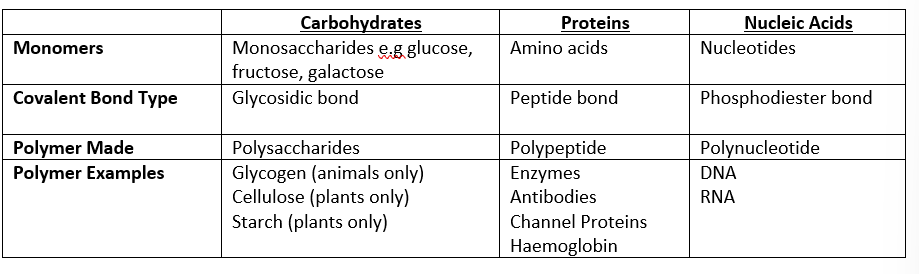
monomer, covalent bond type, polymer made and polymer example for: carbohydrates, proteins and nucleic acids
protein functions
main component of many cellular structures
form enzymes and chemical messengers
amino acid→polypeptide(peptide bond)
nucleic acids functions
form polymers(DNA and RNA) that make up genomes of organisms and code for the sequence of amino acids to make proteins
neucleotides→polyneucleotides (phosphodiester bond)
lipids functions
used as respiratory substrates providing energy for cells
form a bilayer in cell membranes and makeup some hormones
not polymers because made of different smaller units, not one repeating smaller unit
glycerol + fatty acids→lipids (ester bond)
carbohydrates functions
respiratory substrate providing energy for cells
can be used for structure in cell membranes & cell walls in plants
monosaccharides→polysaccharides (glycosidic bond)
bonds(in order of strength)
hydrogen: weakest
electrons are unevenly distributed in some molecules so some regions are - and others are _
this is polarised/a polar molecule
there are weak electrostatic forces of attraction between the + and - regions of polar molecules, especially in water
ionic:
ions with oppositite charges(+ metal and - non metal) attract eachother.
the electrostatic force of attraction is called an ionic bond
covalent:
atoms share electrons in the outershell to make a more stable compound with a full outershell, called a molecule
the bond is the electrostatic force of attraction between + nuclei and - shared electrons
valency of: carbon, oxygen, hydrogen and nitrogen
valency- number of bonds it can form
carbon-4
nitrogen-3
oxygen-2
hydrogen-1
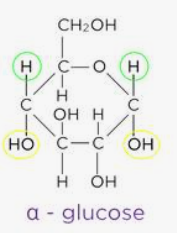
alpha glucose
an isomer of glucose that can bond together to form starch or glycogen
forms a helical chain→compact→more can be stored
beta glucose
an isomer of glucose that can bond together to form cellulose
forms long straight unbranched chains bc each B-glucose molecule is inverted so all 1-4 glycosidic bonds
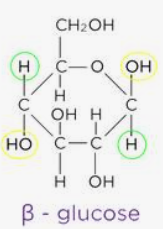
isomer
compounds with the same formula but different structure
monosaccharides
general formula is (CH2O)n
monomers that make up disaccharides and polymers
e.g. fructose(in sweets, sweetest, most soluble so more water can enter), glucose, galactose(least soluble)
hexose sugar- 6 carbons e.g glucose, pentose sugar- 5 carbons
disaccharides
2 monosaccharides joined by a condensation reaction forming a glycosidic bond between two -OH groups
reducing sugars can lose/donate electrons to other compounds
maltose(reducing)= glucose + glucose
lactose(reducing) = glucose + galactose
sucrose(non-reducing) = glucose + fructose
enzymes catalyse hydrolysis to break di→mono e.g lactase for lactose→glucose +galactose
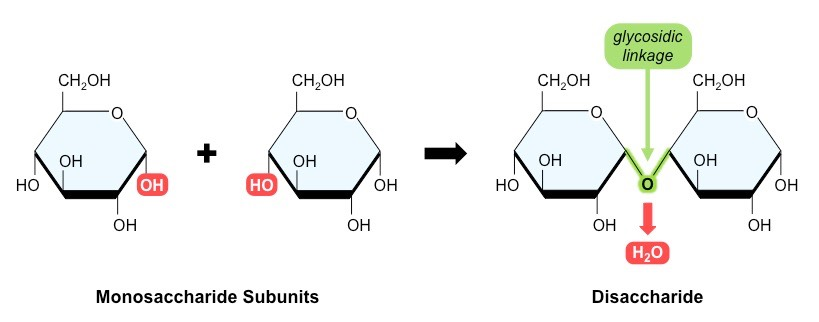
polysaccharides
more than 2 monosacharides joined by condensation
polymers of glucose are joined by either a 1-4 or 1-6 glycosidic bond(1-6 means carbon 1 of first glucose joins to carbon 6 of the second glucose) causing different shapes & properties
amylose and cellulose- unbranched glucose chains w 1-4 glycosidic bonds
amylopectin and glycogen- branched w 1-6 glycosidic bonds
starch: structure, properties, uses, and diagram
monosaccharide- alpha glucose
structure:
mixture of polysaccharides: amylose and amylopectin
amylose-long unbranched, 1-4 glycosidic bonds, helical so coiled due to H+ bonding
amylopectin- long, branched chains bc of 1-6 glycosidic bonds
properties:
amlose-coiled so its compact to store more in a smaller space
amylopectin- branches increase surface area→faster hydrolysis→more glucose released for respiration
uses:
plants- store excess glucose as starch in grains & granules bc starch is too large to leave the cell and is insoluble so doesnt affect water potential(amount of water in/out of cell) and is hydrolysed by amylase and maltase for glucose for respiration
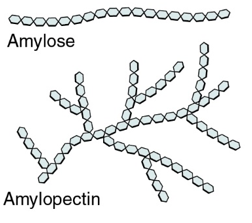
glycogen: structure, properties, uses, and diagram
monosaccharide- alpha glucose
structure:
long branched chain w 1-6 glycosidic bonds and lots of long side branches(more than amylopectin)
properties:
lots of branches increase surface area for enzymes to hydrolyse glycosidic bonds for quick release of glucose. also compact→good for storage
less dense & more soluble than starch→broken down quicker
uses:
animals store excess glucose as glycogen in the liver and muscles in granules that can quickly be hydrolysed to release glucose for respiration when needed e.g during exercise
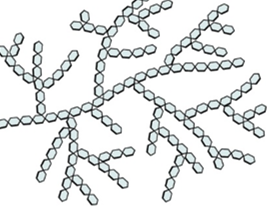
cellulose: structure, properties, uses, and diagram
monomer- beta glucose
structure:
long unbranched, straight chains, 1-4 glycosidic bonds
the cellulose chains are then linked by hydrogen bonds between the glucose molecules in each chain to form thicker fibres called microfibrils
each B-glucose is inverted to the ones either side of it so the chains are straight
hydrogen bonds between each parallel chain
properties:
hydrogen bonds between cellulose chains make the microbrils very strong but still flexible, allowing them to provide support
uses:
plant cell walls use cellulose as a structural component as it provides support and allows cell walls to become turgid
large gaps between microfibrils so substances can move to cell membranes for selective absorption
testing for reducing sugars
all monosaccharides and some disaccharides e.g maltose, lactose
method:
add excess benedict’s reagent to sample
heat in a water bath at boiling/above 80c
result:
blue precipitate(none/orignal colour) →green(trace amounts)→yellow(low)→orange(moderate)→brick red(high)
quantitative measurements:
filter solution and weigh precipitate or remove precipitate and use a colourimeter to measure absorbance of remaining benedict’s solution
how to test for non reducing sugars
some polymers and disaccharides e.g sucrose
method:
add dilute HCl and heat in a water bath to break bonds and produce monosaccharides
add an alkali to neutralize it e.g NaOH
follow method for reducing sugars
hazards and precautions for testing for sugars
benedicts reagent is an irritant→wear goggles, wash hands if contact w skin
hot water can cause burns→use caution when carrying or pouring
(non reducing only) HCl and alkalis e.g NaOH can be corrosive→ wear goggles, wash hands if contact w skin
testing for starch
add iodine
if starch is present it turns from brown to blue-black
this is qualitative only
uses of lipids
electrical insulation for nerves and thermal insulation in adipose(fat) cells
steroid hormones e.g testosterone
forms the plasma membrane
protect delicate organs
waterproofing and buoyancy→lipids are less dense than water so float
triglycerides
1 glycerol + 3 fatty acids
properties:
non polar and hydrophobic(repels water) →insoluble in water but soluble in organic(carbon containing) substances e.g ethanol
in water triglycerides bundle together as insoluble droplets bc the tails face inwards and the glycerol heads shield them from water
ester bonds form between each glycerol’s OH group and each fatty acids’s OH group through a condensation reaction also called esterification
triglycerides are stored in adipose(fat) tissue and are used as an energy store bc lots of energy is released when fatty acid chains break down
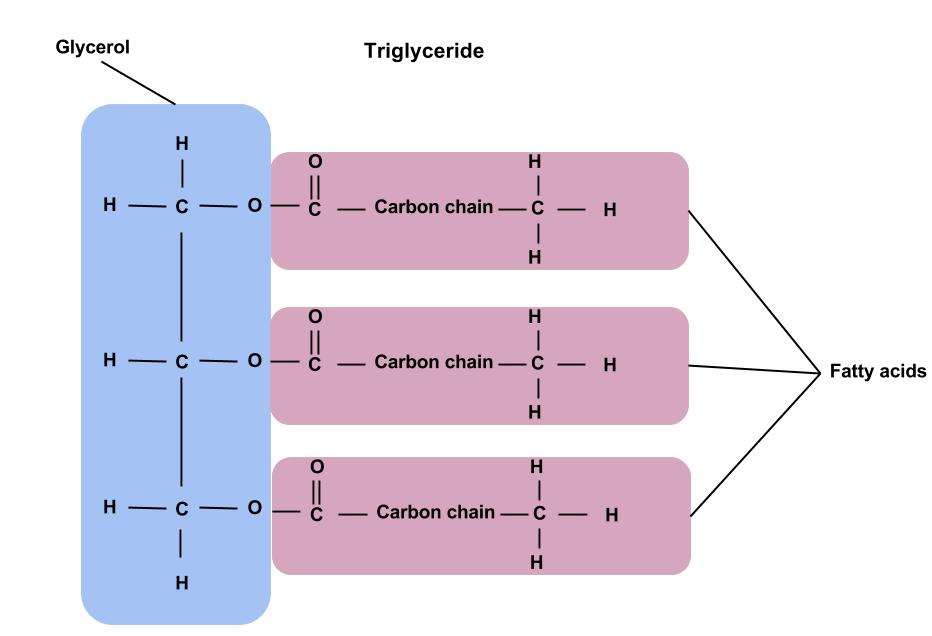
mono and diglycerides
monoglycerides- 1 fatty acid+ 1 glycerol
diglycerides- 2 fatty acids+ 1 glycerol
fats vs oils, saturated vs unsaturated fatty acids
fat-saturated, solid at room temp, higher density
oil-(poly)unsaturated, liquid at room temperature, less dense
saturated-no double bonds between carbon atoms in the hydrocarbon chain
unsaturated-has double bonds between carbons in the hydrocarbon chain so fewer hydrogen atoms. Double bonds cause the chain to kink/bend so its less dense, can be one-monounsaturated or many carbon double bonds-polyunsaturated
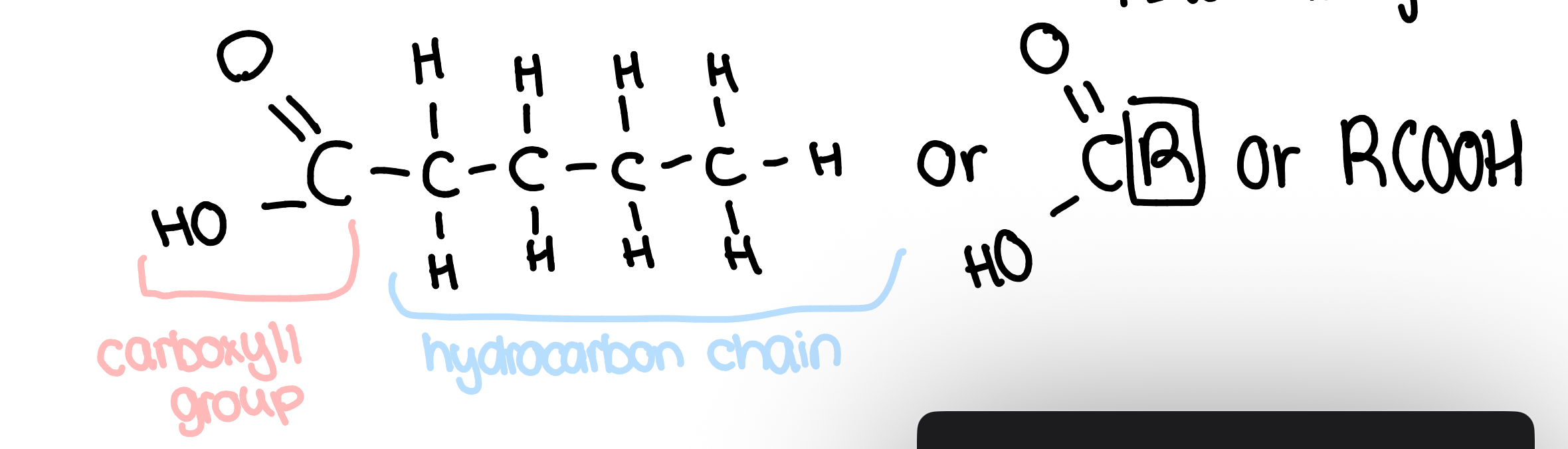
fatty acids
the -OH reacts w glycerol
the hydrocarbon chain is non-polar and hydrophobic
the variable region can vary in length of hydrocarbon chain, or if it is saturated or unsaturated
phospholipids
glycerol + phosphate + 2 fatty acids
phospholipids are polar- 2 ends/poles that behave differently. Because phosphate is hydrophyllic, the head is attracted to water but the tail is hydrophobic so repels water
hormones are phospholipids
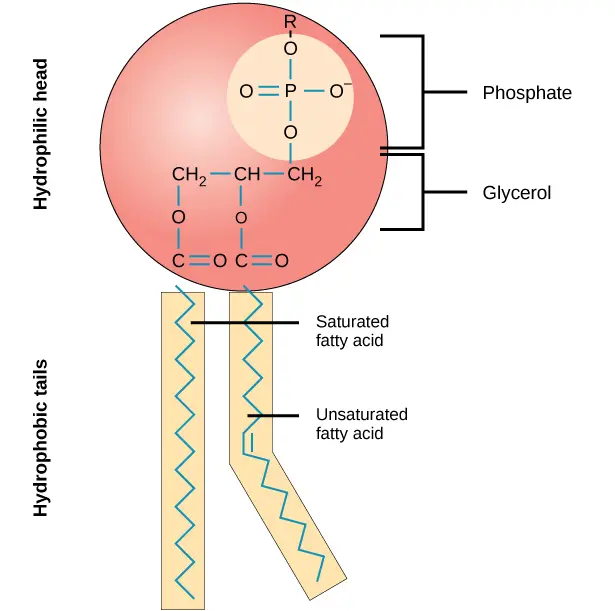
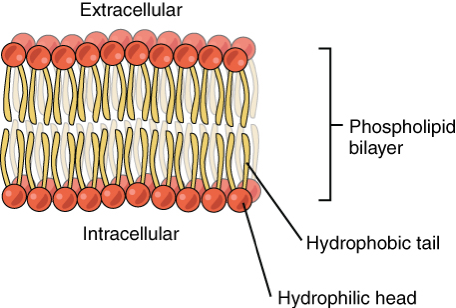
phospholipid bilayers
When water is in(intracellular) and out(extracellular)of cells, the hydrophobic tails move inwards and the hydrophyllic heads move outwards forming a double layer called a bilayer, making cell membranes.
The center is hydrophobic so water soluble molecules can’t easily enter, creating a barrier separating solutions and creating different conditions either side of the membrane
This structure allows the phospholipid to form glycolipids by combining w carbohydrates within the cell-surface membrane. Glycolipids are important in cell recognition
micelles
phospholipid structure that forms when water surrounds it, but there is no water inside
can be used to transport monoglycerides

testing for lipids
emulsion test
1. add ethanol to sample(bc insoluble in water) and shake
2. add water to sample and shake for a minute
positive-lipids form a milky white emulsion(layer at the top)
more lipids present=more milky/opaque emulsion but qualitative only
ethanol is flammable so do not conduct the test near open flames
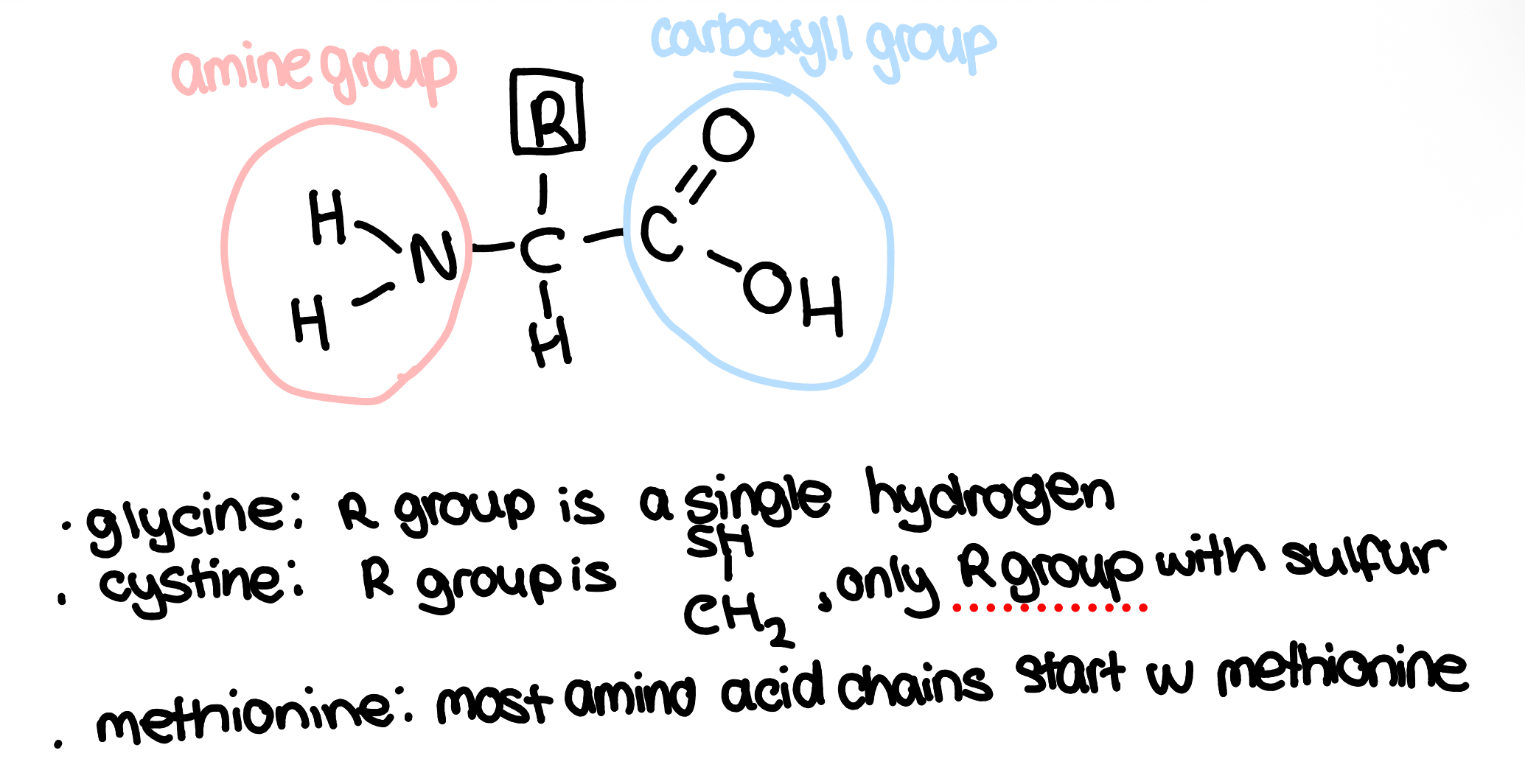
amino acids
proteins are made of the monomer amino acids
there are only 20 possible amino acids but if the amino acids are in a different sequence, the (disulfide bridges/hydrogen/ionic) bonds are in different places so the protein folds in a different way and has different 3D shapes in the tertiary structure so form different proteins
R group is the variable region(only part that changes), it is usually lots of carbon
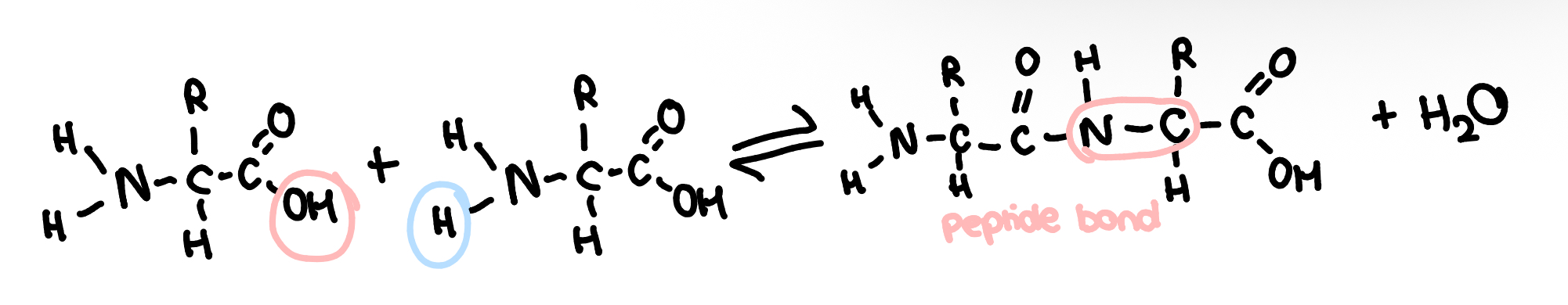
dipeptides
two amino acids joined by a condensation reaction to form a dipeptide w a peptide bond
the bond is between the amine group and the carboxyl group of another
polypeptides
more than 2 amino acids joined together
proteins are folded chains of single or multiple polypeptides
There are 4 levels to protein structure, based on how much it folds/changes shape. Single chain polypeptides can fold to the tertiary level, but only multichain polypeptides can fold to the quaternary level
in all proteins, more bonds=more stable
primary structure
the sequence of amino acids in a polypeptide chain
the number and sequence of amino acids in a polypeptide chain determines its structure and shape
a protein’s shape is specific to its function so change in amino acids→changes shape→can’t function
secondary structure
coil spiral e.g alpha/beta helice or a beta pleated sheet
amino acids in the chain have -NH and -C=O groups on either side of the peptide bond
the H in -NH has a + charge and the O in -C=O is - charged
both groups form weak hydrogen bonds causing the polypeptide chain to be twisted into a 3D shape
tertiary structure
the secondary structure twists and folds even more, into a 3D globular stucture e.g enzymes
the 3D shape is what makes each protein distinctive and recognisable to other molecules to interact in specific ways
their bonds depend on the primary structure. The 3 possible bonds are:
disulfide bridges- fairly strong and not easily broken
ionic bonds-form between any carboxyl and amine groups that are involved in peptide bonds, weaker than disulfide bridges and easily broken by changes in pH
hydrogen bonds-numerous but easily broken
quaternary structure
multiple polypeptide chains linked together to form compelx molecules
haemoglobin has haem groups that are prosthetic(non protein) and contains ferrous iron that binds to oxygen. Haemoglobin has 2 alpha helices/chains and 2 beta helices/chains
fibrous proteins
e.g collagen
structural functions such as tendons to join muscles to bones
long chains that run parallel to eachother. Chains are linked by cross-bridges so they form very stable molecules w a high tensile strength
collagen has 3 alpha helice chains
biuret’s test
detects the peptide bonds in protein
add buiret’s agent to sample
if proteins/peptide bonds are present it turns purple. If not, the solution remains blue
how do enzymes work
enzymes are globular 3D catalysts that act as biological catalysts to speed up the rate of metabolic reactions without being used up
They decrease activation energy to allow reactions to happen at lower temperatures
depending on the reaction, they either hold substrates close together, reducing repulsion and allowing them to bond more easily or put more strain on bonds allowing them to break apart more easily
lock and key model
active site is always complementary to substrate
acrive site is rigid(disproved bc other molecules can bind to different sites to the active site on enzyme and alter the enzymes shape and activity-so active site must be flexible)
basic explanation, doesn’t show how activation energy is lowered
induced fit model
active site isn’t complementary at first, but undergoes a confirmational change to become complementary
active site is flexible and moulds around the shape, putting strain around bonds to decrease activation enregy
more complex and more widely accepted explanation
intercellular vs extracellular
anabolic and catabollic
intercellular- in cells e.g hydrogen peroxide→oxygen + water, enzyme: catalase
extracellular- outside cells e.g protein→amino acids enzyme: trypsin
catabollic reaction- breaks down.e.g. hydrolysis
anabolic reaction-builds e.g condensation
graph showing rate of reaction as substrate concentration increases with no inhibitor, a competitive inhibitor and a non-competitive inhibitor
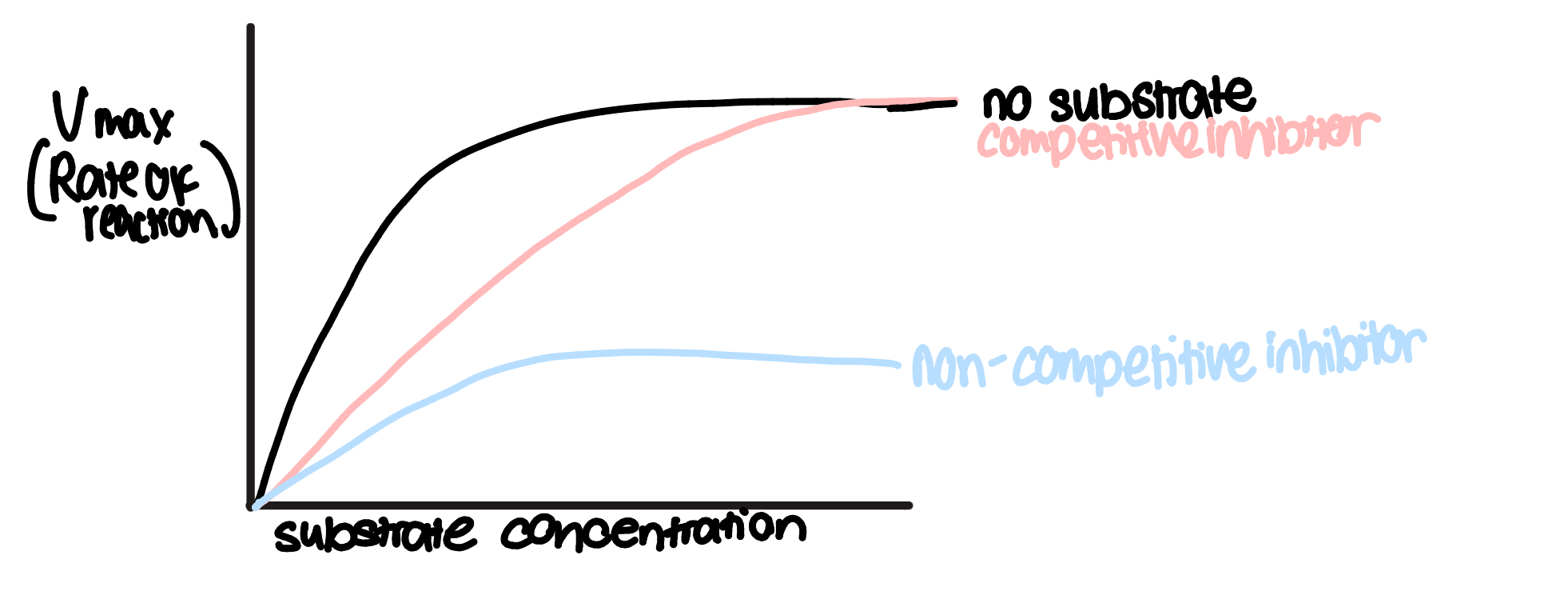
competitive inhibitors
competitive inhibitors are molecules that have a similar shape to a substrate, so they can occupy the active site of an enzyme
therefore, they compete w substrates for available active sites
increasing substrate conc reduces the effects of competitive inhibitors by reducing the chance of inhibitors colliding w active site
some competitive inhibitors bind irreversibly(permanently) to an active site
e.g. succinate is a substrate for an enzyme in respiration but malonate has a similar structuer and can be a reversible competitive inhibitor
e.g. pencilin is an irreversible competitive inhibitor to transpeptidase- an enzyme for the synthesis of bacterial cell walls, so is used to treat bacterial infections
non-competitive inhibitors
binds to enzyme’s allosteric site
this causes the enzyme’s tertiary structure to undergo a confirmational change in shape, so active site changes
so the active site is no longer complementary to substrate so it can’t bind to form enzyme-substrate complexes
This reduces rate of reaction and unlike with competitive inhibitors, its effects cannot be overcome by increasing substrate concentration
enzyme cofactors
non protein structures that bind to active site to make it complementary to a substrate
co-enzymes- e.g NAD, FAD, NAPD→gain electrons to accept hydrogen to assist in reactions, organic molecules, often from vitamins e.g vitamin B5 is used to make coenzyme A used in aerobic respiration, temporarily bind to enzymes
inorganic cofactors- don’t contain carbon, usually metal ions
prosthetic groups- tightly bound cofactors that permanently attach to enzymes, e.g zinc ions are a prosthetic group for carbonic anhydrase- an enzyme to regulate pH in blood
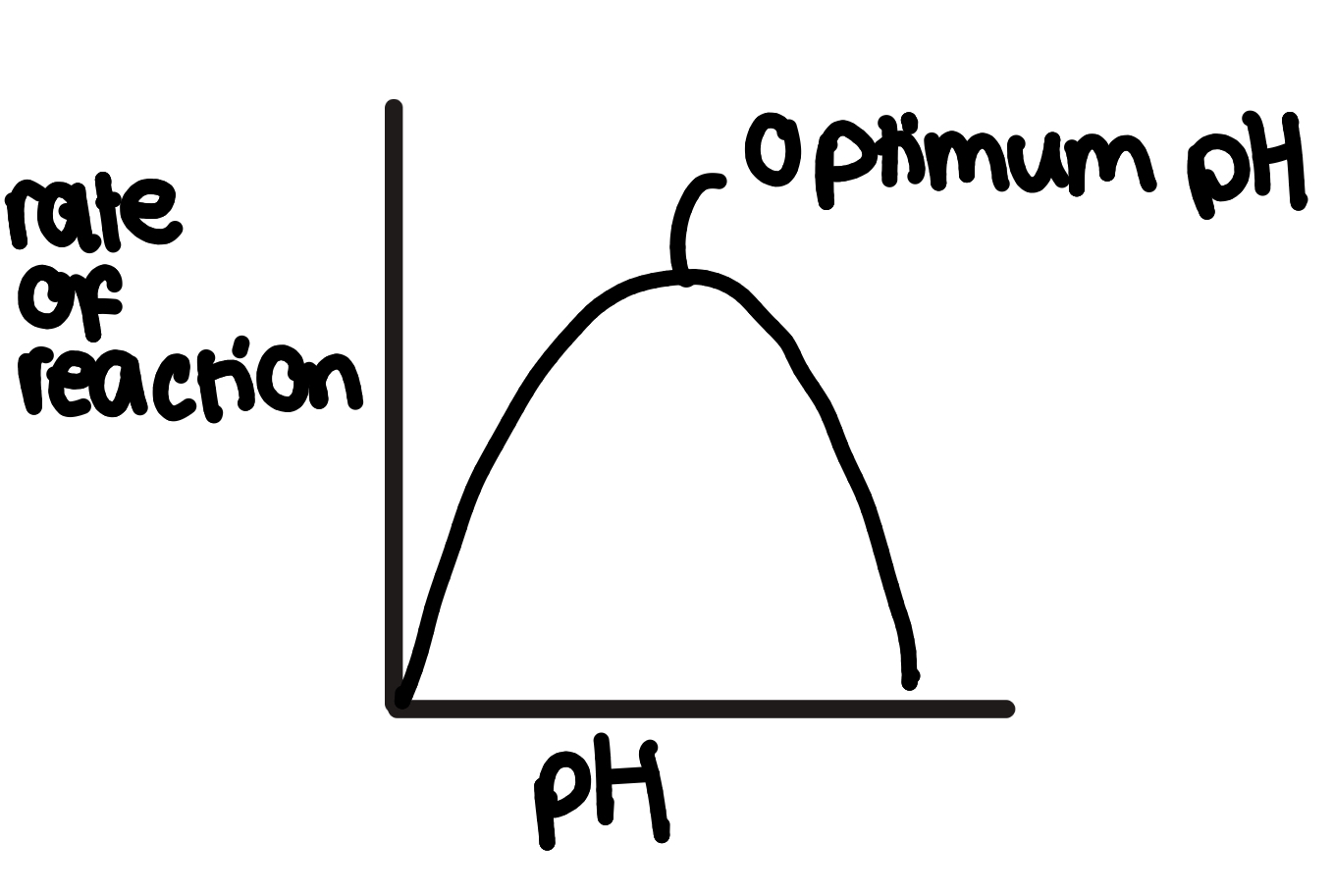
how does pH affect enzyme action
pH is the measure of H+ ions in a solution
the shape/arrangement of an enzyme’s active site is partly determined by the hydrogen and ionic bonds in the enzyme’s tertiary structure
the change in H+ ions affects this bonding so
outside of optimum pH the hydrogen and ionic bonds holding the enzyme’s tertiary structure together break so it unfolds and the enzyme denatures
so the active site is no longer complementary to the substrate, so enzyme-substrate complexes can’t form, reducing the rate of reaction
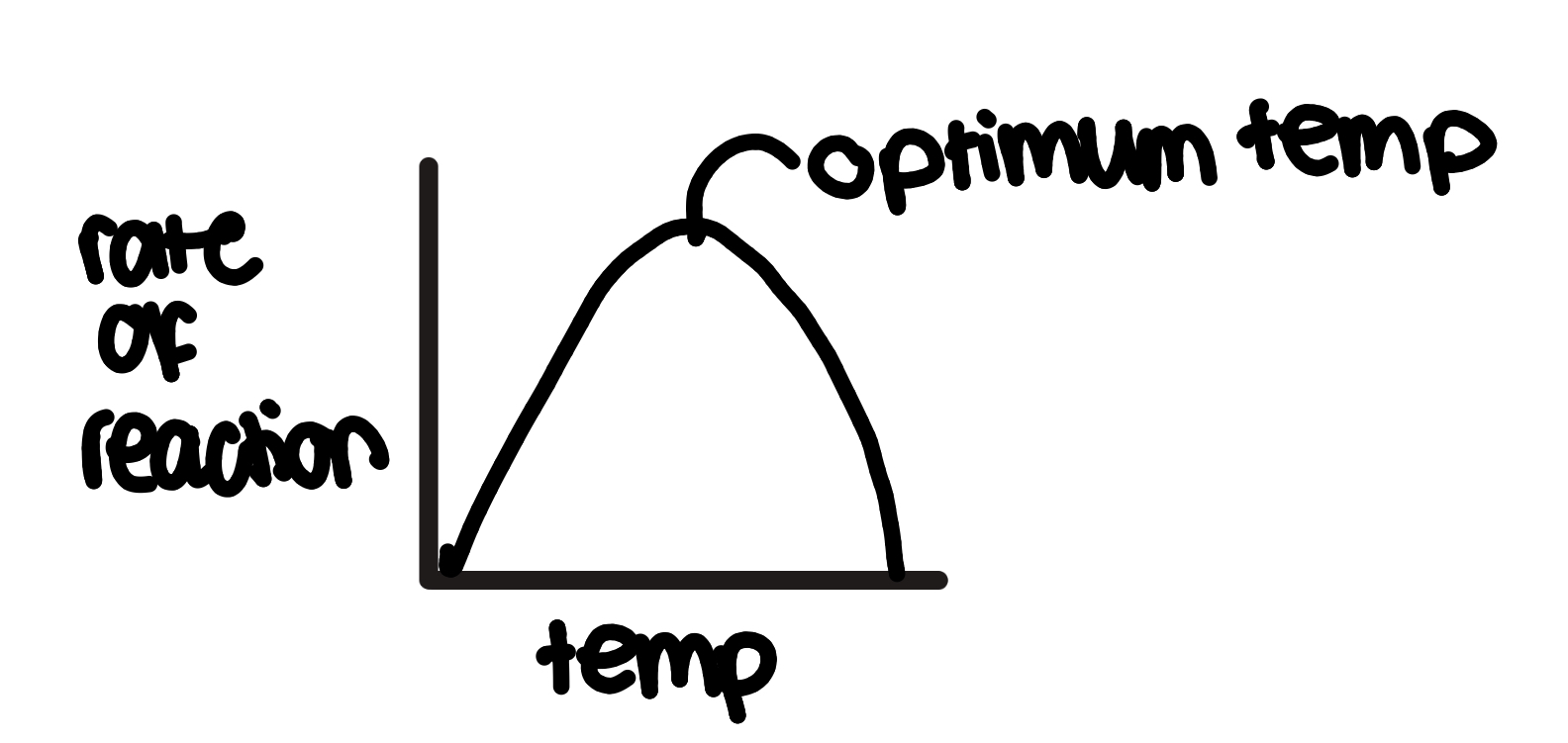
how does temperature affect enzyme action
at first, increasing temperature increases rate of reaction
bc particles have more kinetic energy so move faster
so more frequent successful collisions, so more enzyme-substrate complexes formed
but past optimum temperature:
increasing temp decreases rate of reaction bc there is too much kinetic energy so
hydrogen bonds in tertiary structure of active site break and the enzyme unfolds
the active site changes shape and is no longer complementary to substrates since the enzyme is denatured
so fewer enzyme-substrate complexes are made
how does concentration of enzyme/substrate affect enzyme action
1:
increasing substrate concentration increases the rate of reaction bc there are more frequent successful collisions and the substrate is the limiting reactant
can replace substrate for enzyme
2: the rate of reaction is limited by:
number of enzymes available(if increasing substrate conc) so all active sites are saturated by substrates so it plateaus
number of substrates available(if increasing enzyme conc) so not enough substrates to supply enzyme’s active sites so it plateaus

how to measure rate of reaction
draw a tangent with an equal number of squares either side
change in y/change in x
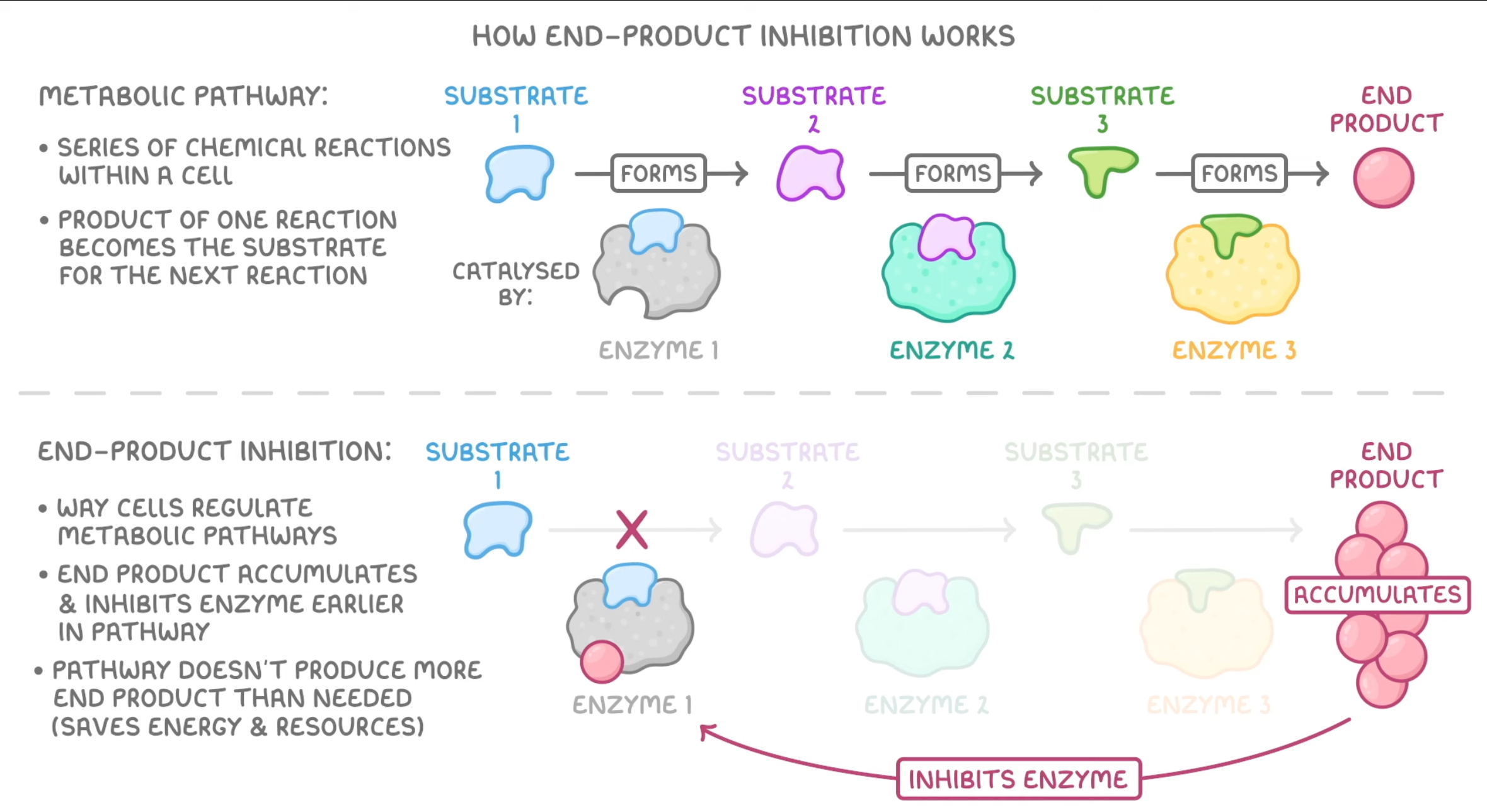
end point inhibition/feedback inhibition
water properties overview
is a major compontent of cells
is a metabolite(necessary for metabollism)
is a solvent
has a large latent heat of vaporisation
has a high specific heat capacity
has strong cohesion between molecules
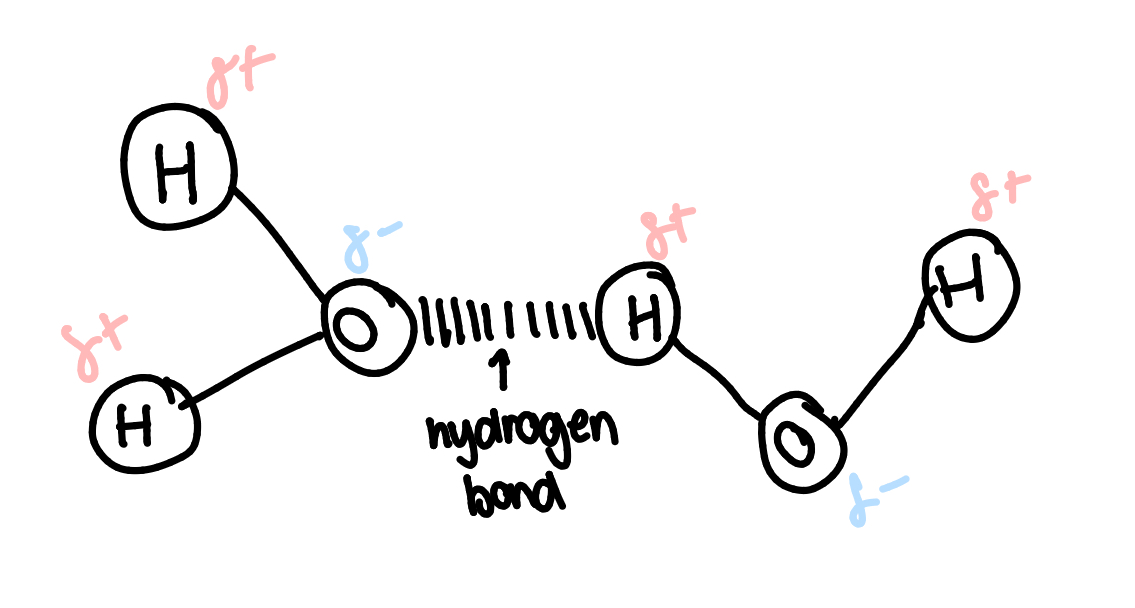
water structure
2 hydrogen atoms covalently bonded to 1 oxygen
polar molecule- electrons aren’t equally shared(hydrogen is slighly +, O is slighly -)
dipolar
hydrogen bonds in water:
the slightly negative charge on the oxygen atom attracts it to the slightly positive hydrogen atom of another water molecule
many of the properties of water are due to its ability to form hydrogen bonds
the numerous hydrogen bonds in water make it a very stable structure
water’s specific heat capacity
the energy to raise the temperature of 1kg of water by 1 degree
water has a high specific heat capacity because water molecules stick together bc of the hydrogen bonds so more energy is needed to seperate them
this means water can act as a buffer against sudden temperature variations
this is useful bc organism’s bodies are mostly made of water. The water in and around our cells absorbs a lot of heat energy without its temperature increasing much to ‘bufffer’ heat changes
water’s high SHC is also an advantage to aquatic organisms bc large bodies of water(seas and lakes) don’t change temperature as quickly as terrestrial(land) environment
latent heat of vaporisation
energy required to evaporate 1g of water
water has a high latent heat of vaporization bc there are hydrogen bonds between water molecules which requires lots of energy to break
in a body of water some molecules are moving at faster speeds so some have enough enrgy to escape the water and move into air-evapouration
evaporation causes energy loss, decreasing the kinetic energy of water so it cools
importance to organisms:
animals that sweat can keep cool bc the water in sweat evaporates of the surface of the animal
plants are also cooled when water evaporates from their leave
water potential
water moves from high to low concentration- osmosis
water also tends to move from areas of high hydrostatic pressure to low
water movement is also affected by gravity and electrostatic forces-such as those that cause surface tension
the tendency for water to move due to any of these effects is water potential
water’s cohesion
cohesion- an attractive force between particles of the same kind e.g. water and water
adhesion- an attractive force between unalike substances
water sticks together due to the cohesive forces of hydrogen bonding
this allows it to be pulled up a tube(like drinking through a straw). This happens in xylem vessels
water and surface tension
acts as a force pulling droplets of water back to a body of eater
the water surface acts a skin strong enough to support small organisms e.g pond skaters
cohesion-tension theory
water is a polar molecule, so its positive and negative charges aren’t evenly distributed
the O is slighly - and the H is slighly +
so in the xylem, water molecules spontaneously arrange so the + and - poles lie next to eachother
this causes the molecules to cohere(stick together) so as some leave a plant by transpiration, others are pulled up behind them
water density
when cooled all substances move closer together and become more dense
water is most dense at 4 degrees
below 4c it becomes less dense and forms a crystalline structure. This structure is unique, hence why all snowflakes are different
importance to organisms:
ice is less dense than water so it floats. This insulates lakes and oceans to prevent them from freezing solid, allowing organisms to survive winter. Ice caps are a habitat for polar bears
water as a solvent
a solvent is a liquid that solutes can dissolve in
dissovled solutes are free to move
positive and negative charges of water attract other molecules causing the solute to separate and dissolve
importance:
the metabolic reactions in all organisms only happens when the reactants are dissolved in water
substances being dissolved in water allows them to be transported around the bodies of organisms e.g glucose, CO2 and urea in blood plasma, and ions and sugar dissolved in water are transported by plants phloem and xylem
metabolites
nucleic acids
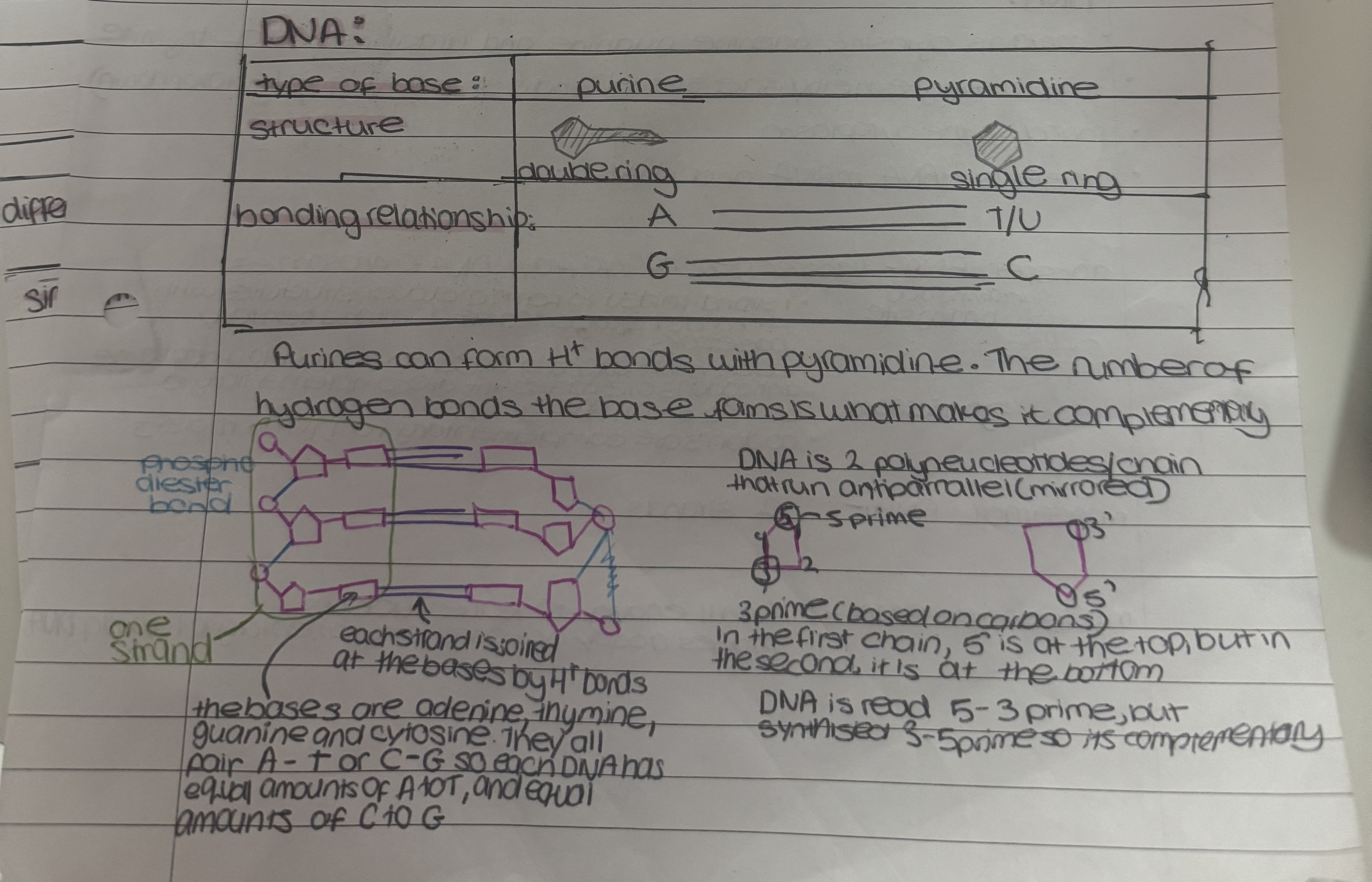
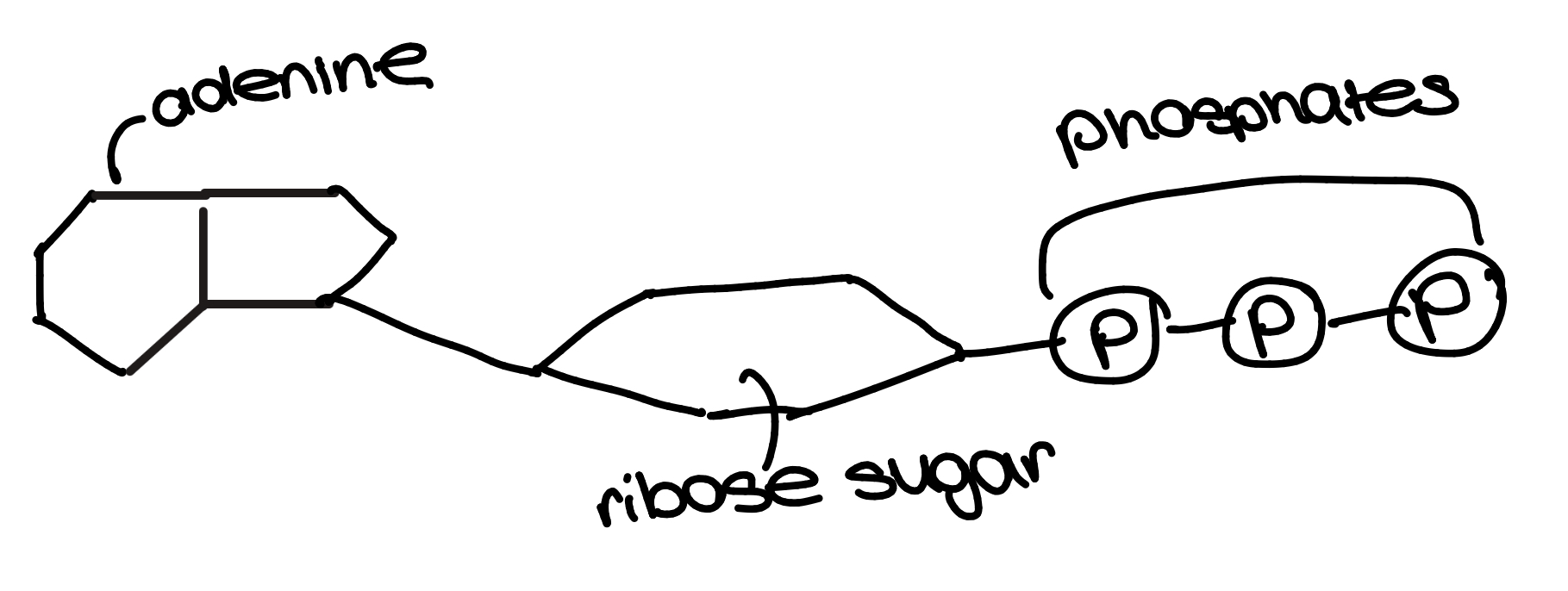
DNA is a nucleic acid. Nucleic acid are macromolecules containing O, H, C, N, and P
nucleic acids are made of nucleotides
the 2 nucleic acids are ribonucleic acid(RNA) and deoxiribonucleic acid(DNA)
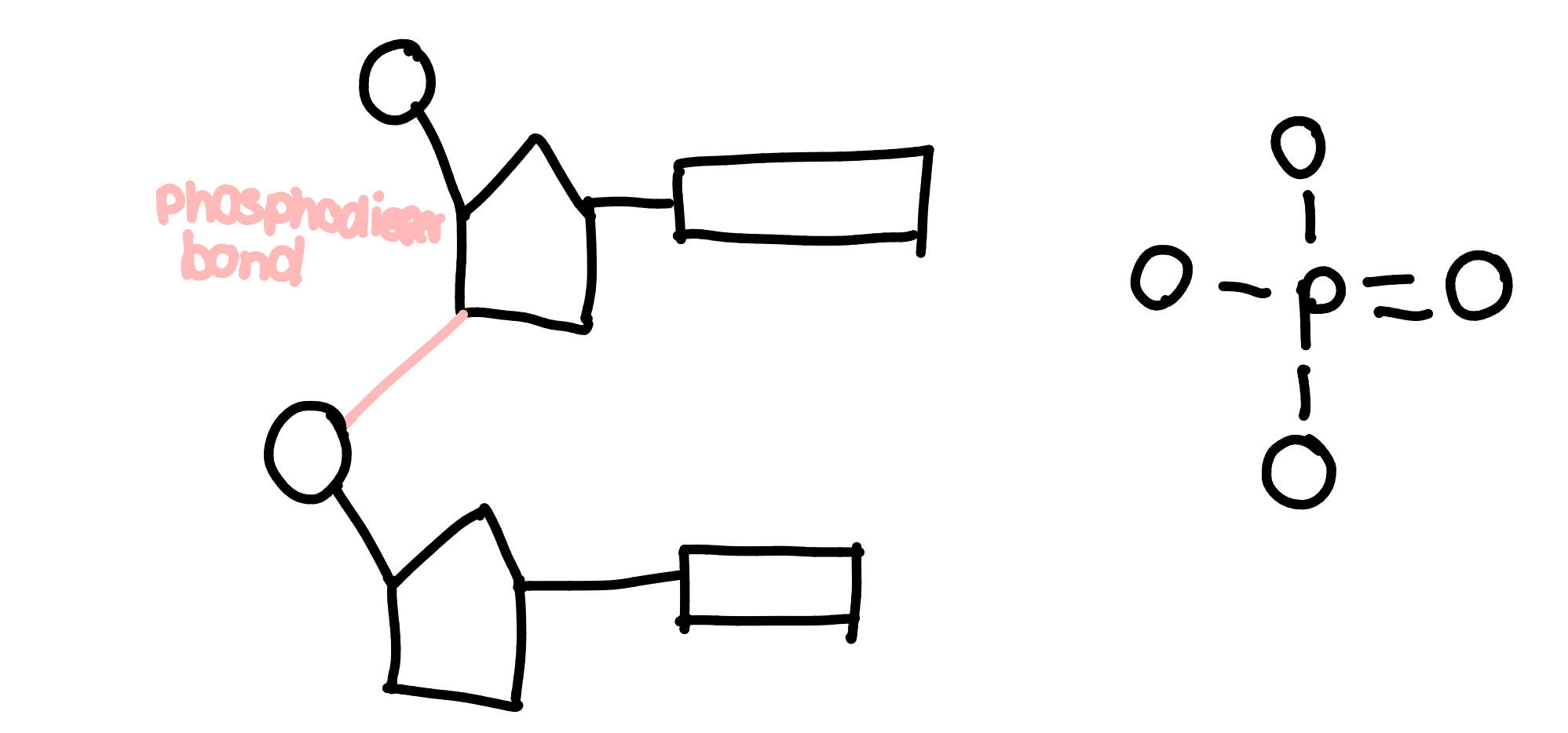
nucleotides
phosphodiester bonds hold nucleotides together
hydrogen bonding in DNA
DNA structure
discovered by Rosalin Franklin
neucleotide chains add stability
long→ store lots of info/entire genome
helix→compact
3.2bn base pairs in DNA→near infinite order→code for different ordeer of proteins→variety for genetic diversity, and also accurate template for replication so identical copies each time
sugar-phosphate backbone→helix structure protects reactive bases
hydrogen bonding→weak so little energy to break apart to act as a template for replication
complementary base pairs→accurate template for replication
RNA
contains cytosine, adenine, guanine and uracil(instead of thymine)
it is a single helix/one strand and is short lived(quickly broken down)
found in the cytoplasm
there is tRNA, mRNA, and rRNA
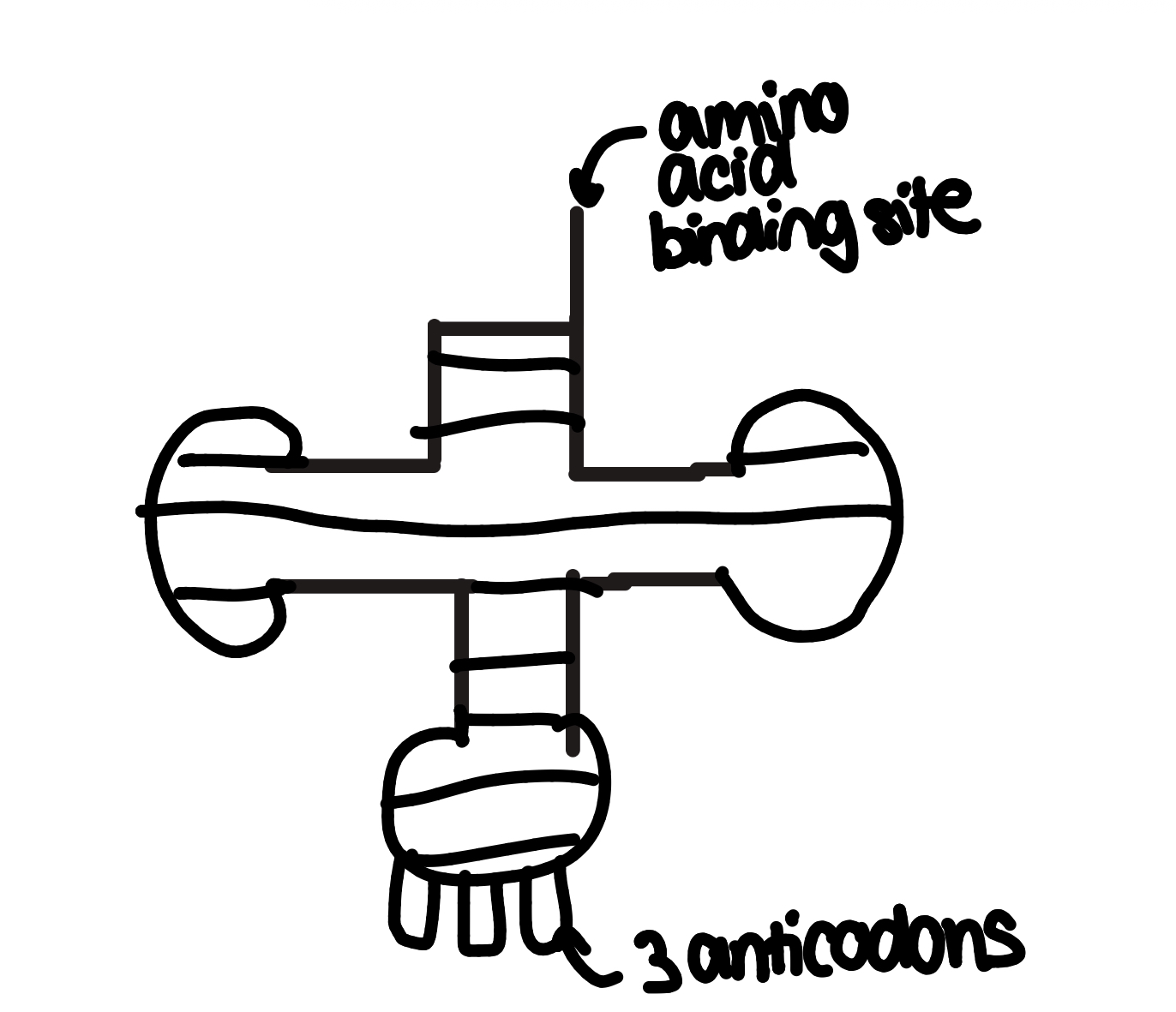
transfer RNA- transfer genetic info from DNA to ribosomes
one strand folded to form a clover structure with H+ bonds between complementary base pairs
each tRNA can bring one specific amino acid to a ribosome
anticodons are bases that are complementary to codons on an RNA strand
codon’s are complementary to DNA triplets
see image
ribosomal RNA- two strands
messenger RNA-
small enough to leave nucleus
makes a copy of bases complementary to one strand of DNA
similarities and differences between DNA and RNA

similarities and differences between rRNA, mRNA, and tRNA

replication theories
DNA has to replicate every time a cell divides so both cells have identical copies of the entire genome. The method of replication is called semi conservative replication
DNA helicase- enzyme that breask down H+ bondes between bases(give example of base in ExamQs)
DNA polymerase- join adjacent neucleotides forming phosphodiester bonds to synthesise the sugar phosphate backbone
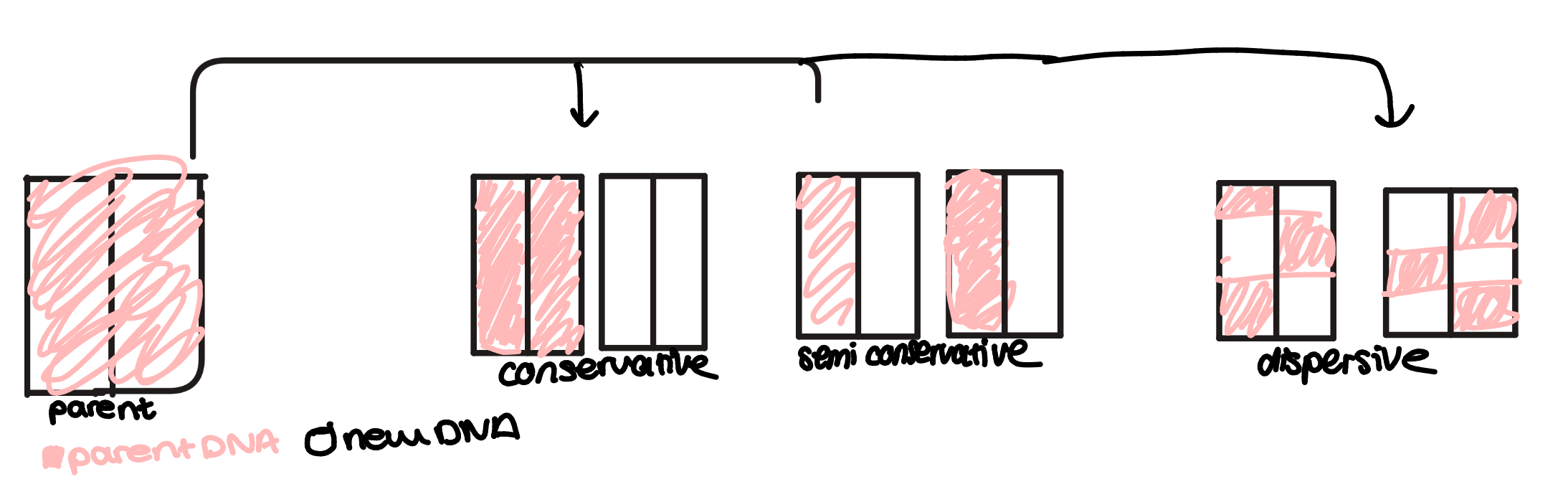
replication steps
the enzyme DNA helicase ‘unwinds’ the double helix by breaking H+ bonds between the bases of 2 DNA strands
each original strand acts as a template determining the order of bases to build a new strand. Free DNA nucleotides are attracted to the exposed bases on the templates and they attach to the correct base through complementary base pairing(A w T, and C w G)
the free nucleotides are joined to the new strand by condensation reactions catalysed by the enzyme DNA polymerase
each new DNA molecule contains one strand from original DNA and one new strand
how does DNA polymerase work
each DNA strand has a directional structure due to the ends of the strand being either a sugar attached to the 5th carbon, 5’(5 prime end), or a hydroxyl group attached to the 3’ end
DNA polymerase is only complementary to the 3’ end of the template strand, so it can only move along the template strand and add nucleotides in the 3’ to 5’ direction so the new strand is built 5’ to 3’ bc the strands are antiparallel
1 strand continously built, whilst the other is built in sections in the opposite direction as DNA is unwound
the DNA polymerase on the opposite template has to detach and reattach so it often moves more slowly
experimental evidence for semi-conservative replication
conservative replication was still a possibility until Melelson and Stahl proved DNA replication was semi conservative
nucleotides contain Nitrogen. They used one light N and one heavy N isotope. DNA samples containing different isotopes can be separated by weight in a centrifuge as heavy DNA sinks and light DNA settles out higher up in the solution
to get samples of light and heavy DNA, they grew 2 cultures of bacteria in nutrient broth containing either light of heavy N so it would be in their nucleotides
then the heavy N bacteria were put in a broth w only light N, so when they replicated the parent strand would contain heavy nucleotides but only be able to use light nucleotides to make the new strand
if replication was semi conservative→Dna is a mixture of heavy and light/ one strand of each so DNA would settle in the middle in the centrifuge solution. This is what happened
The second generation showed that semi conservative replication continued bc a light band was formed. This is because the DNA molecule splits 2 strands would have been formed with the light DNA strand as the template and two would be a mix as they used the original heavy DNA as a template.
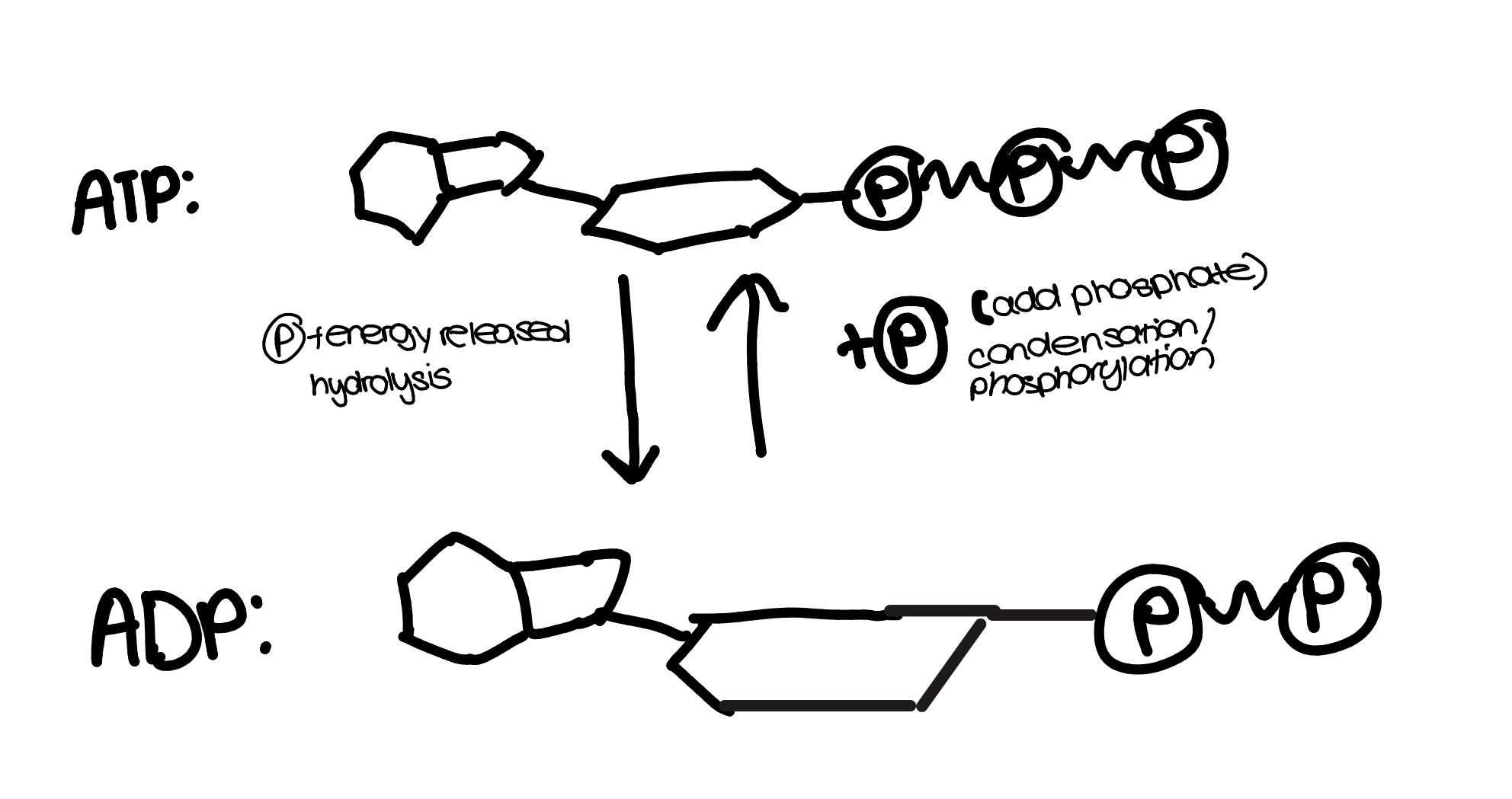
ATP
energy is stored in the bonds between phosphates and released when they are hydrolysed
phosphates that have been separated from ATP can be added to other molecules to make them more reactive(phosphorylating) Dephosphorylation is the opposite
ATP is hydrolysed w ATP hydrolase, and synthesised from ADP by ATP synthisase
endergonic and exergonic reactions
endergonic- absorbs free energy from surroundings
exergonic- spontaneously releases energy for other molecuels
phosphorylation reaction
inorganic ions: hydrogen, iron, sodium, phosphate
inorganic ion- ion that doesn’t contain carbon. They occur in solutions in the cytoplasm and body fluids of organism, in various concentrations
hydrogen ions(H+): determine pH of substances such as blood, the higher the concentration of H+ ions, the lower the pH
Iron ions(Fe2+/Fe3+): a component of haemoglobin-an oxygen carrying molecule in red blood cells
sodium ions(Na+): involved in co-transport of glucose and amino acids
phosphate ions(PO43-): a component in DNA and ATP
method for measuring effect of temperature on rate of enzyme-controlled reactions
make 2 controlled variables:
add 5cm3 milk suspension+5cm3 distilled water to a test tube(no enzyme activity)
add 5cm3 milk suspension + 5cm3 HCl to a test tube(completely hydrolysed sample)
In another 3 test tubes add 5cm3 milk to each and place in a water bath at 10c for 5 minutes to equilibirate
add 5cm3 trypsin to each test tube at the same time and immediately start the timer
record how long it takes for the milk samples to completely hydrolyse and turn colourless
repeat steps 2-4 at 20c,30c,40c,50c, and 60c
find the mean time for milk to hydrolyse at each temperature and work out the rate of reaction rate of reaction= 1/mean time
milk contains a protein called casein which causes the milk to turn colourless when broken down. Trypsin is a protease enzyme to hydrolyse casein.