Renal Function
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
90 Terms
Core Roles of the Kidneys
Urine formation
Fluid and electrolyte balance
Acid–base regulation
Excretion of waste (esp. nitrogenous waste from protein metabolism)
Drug and toxin elimination
Hormone secretion:
Renin → blood pressure regulation
Erythropoietin → RBC production
1,25-dihydroxyvitamin D₃ → calcium regulation
Prostaglandins → local vasodilation, perfusion support
Purpose of Kidney Function Testing
Evaluate renal disease
Assess hydration status (water balance)
Detect acid–base imbalances
Monitor renal impact in:
Trauma
Head injury
Surgery
Infectious disease
Glomerular Filtration — Structure & Selectivity
Site: First part of nephron (glomerulus)
Function: Filters incoming blood
Facilitating Factors:
High glomerular capillary pressure
Semipermeable basement membrane (~66 kDa cutoff ≈ albumin)
Negatively charged membrane repels proteins
Filtered: Water, electrolytes, glucose (partly reabsorbed), amino acids (completely reabsorbed), LMW proteins, urea, creatinine
Excluded: Albumin, large plasma proteins, cells, lipids, bilirubin
Glomerular Filtration — Rate & Clinical Utility
Renal blood flow: 1200–1500 mL/min
Filtrate produced: 125–130 mL/min (protein- & cell-free)
Key metric: Glomerular Filtration Rate (GFR)
Essential for assessing renal function
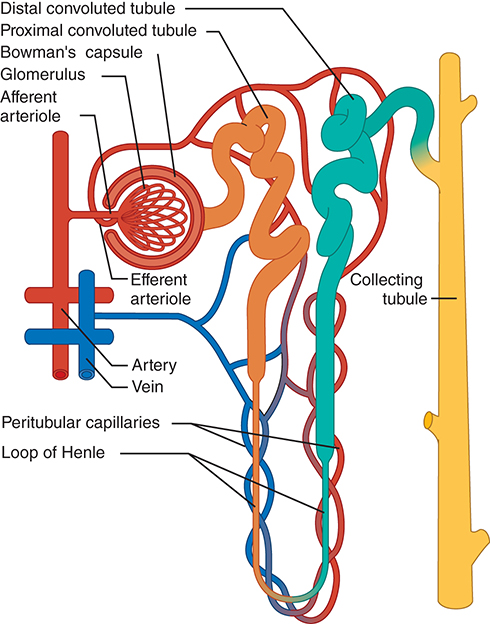
Renal Tubular Anatomy Summary
Glomerulus (Bowman's Capsule)
• Located at the start of the nephron
• Filters blood plasma into filtrateProximal Convoluted Tubule
• In the renal cortex
• Major site of reabsorption of water, electrolytes, nutrientsLoop of Henle
• Descends into medulla, then ascends back
• Establishes medullary concentration gradient (via countercurrent mechanism)
• Descending limb: permeable to water
• Ascending limb: permeable to Na⁺/Cl⁻, impermeable to waterDistal Convoluted Tubule
• In the cortex
• Fine-tunes electrolyte and pH balanceCollecting Duct
• Formed from multiple distal tubules
• Final site of water/electrolyte regulation, responsive to ADH/aldosterone
Tubular Physiology Overview
3 Core Renal Processes
• Glomerular filtration: blood → tubule
• Tubular reabsorption: tubule → blood
• Tubular secretion: blood → tubuleSubstance Handling
• NH₃ (Ammonia): filtered & secreted, not reabsorbed
• Glucose: filtered, partially reabsorbed (reabsorption saturates if threshold exceeded)
• Amino acids: filtered & completely reabsorbed
PCT – Filtrate Reception & Content
Receives filtrate from glomerulus (cell- and protein-free)
• Filtrate contains both waste and valuable solutes
PCT – Reabsorption Function
Returns valuable solutes (glucose, amino acids, ions) to blood via active transport
• Water follows passively (except for chloride, which also moves passively)
• Renal threshold = concentration beyond which solutes appear in urine
PCT – Secretion Function
Moves substances from peritubular capillaries → tubular lumen
• Also secretes metabolic waste from tubule cells into filtrate
Loop of Henle Role
Hairpin loop between PCT and DCT
• Maintains medullary hyperosmolality via countercurrent multiplier
• Descending limb: water reabsorbed
• Ascending limb: Na⁺ & Cl⁻ reabsorbed (impermeable to water)
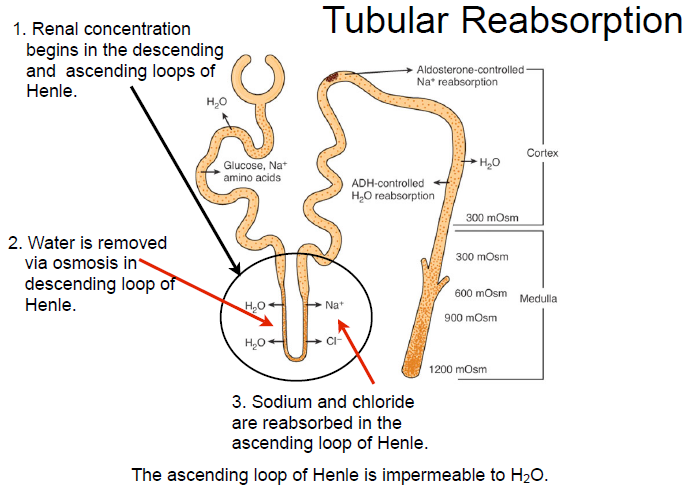
Distal Convoluted Tubule Function
Shorter segment; final adjustments to filtrate
• Key site for electrolyte balance & acid-base fine-tuning
Hormonal Regulation
Aldosterone
Source: Adrenal cortex
Stimulus: Renin–angiotensin mechanism
Effect:
Increases sodium reabsorption
Promotes potassium and hydrogen ion secretion
Acts primarily on distal tubule
Hormonal Regulation
AVP (ADH)
Full Name: Arginine Vasopressin (aka ADH)
Source: Posterior pituitary
Stimulus: Increased plasma osmolality
Effect:
Increases water reabsorption
Makes distal tubule & collecting duct walls permeable to water
Hormonal Response Cascade
↓ Blood pressure/volume → Renin release → Angiotensin II → Aldosterone → ↑ Na⁺ reabsorption → ↑ blood volume
↑ Osmolality → Hypothalamus → Posterior pituitary → AVP → ↑ water reabsorption → ↓ osmolality
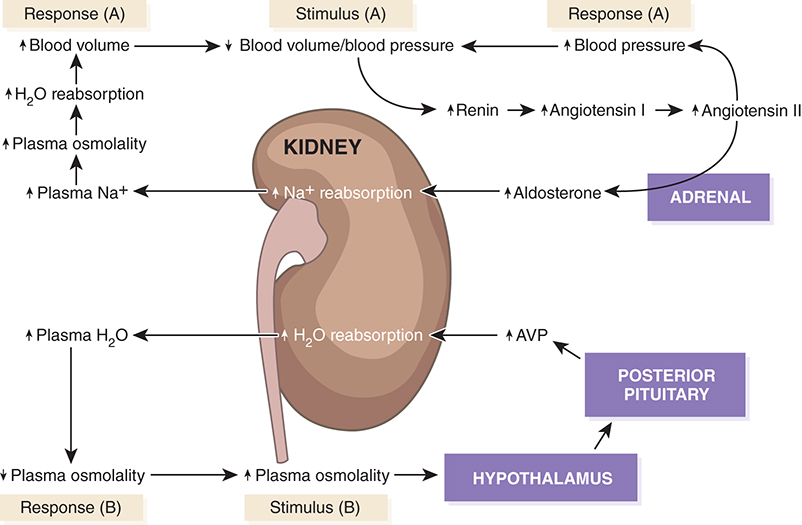
Collecting Duct Function
Final site for urine concentration/dilution
Hormonal targets:
AVP: ↑ water reabsorption
Aldosterone: ↑ sodium reabsorption
Also reabsorbs: chloride and urea
Urea: helps maintain medullary hyperosmolality
Ducts are highly permeable, especially under hormonal influence
NPNs Overview
Definition: Waste products of protein & nucleic acid metabolism
Sources: Degradation of nucleic acids, amino acids, proteins
Key Compounds:
Urea
Creatinine
Uric acid
Urea/BUN
Major NPN (>75%)
Synthesized in the liver from ammonia (protein → amino acids → ammonia → urea)
Prevents toxicity by converting toxic ammonia
Filtered by glomerulus
40–60% reabsorbed in collecting ducts
Creatinine Metabolism
Derived from muscle creatine phosphate via creatine kinase (first muscle fuel source)
~20% of muscle creatine → creatinine daily
Amount produced correlates with muscle mass
Filtered by glomerulus, not reabsorbed
Uric Acid Excretion
End product of purine metabolism
Freely filtered at glomerulus
Undergoes both reabsorption and secretion in tubules
Final excretion: only 6–12% of filtered load
Build up can lead to gout
Water Balance Regulation
Controlled by AVP (arginine vasopressin) in response to ↑ plasma osmolality
Dehydration → max H₂O reabsorption in renal tubules (up to 1200 mOsm/L)
Water excess → excretion of dilute urine (as low as 50 mOsm/L)
Hypothalamus stimulates thirst and AVP release
Fine-tunes fluid status between extremes
Sodium Role in Renal Balance
Primary extracellular cation
Balance controlled solely by excretion
Regulated via renin-angiotensin-aldosterone system (RAAS)
Sodium reabsorption influenced by aldosterone
Potassium Role in Renal Balance
Primary intracellular cation
Competes with H⁺ in exchange for Na⁺
Filtered by the glomerulus and reabsorbed
Plays a role in acid-base and electrical balance
Chloride Role in Renal Balance
Principal extracellular anion
Maintains extracellular fluid volume and osmotic pressure
Passively reabsorbed with Na⁺
Phosphate Role in Renal Balance
Predominantly intracellular anion
Exists in protein-bound and non-protein-bound forms
Higher concentration inside cells than outside
Renal handling regulated by parathyroid hormone (PTH)
Calcium Role in Renal Balance
Second most abundant intracellular cation
Circulates in protein-bound and non-protein-bound forms
Non-protein-bound fraction can be ionized or unionized
Ionized calcium is freely filtered by the glomerulus
Magnesium Role in Renal Balance
Major intracellular cation
Acts as enzyme cofactor
Exists in both protein-bound and ionized forms
Ionized magnesium is filtered and reabsorbed by the kidney
Renal Role in Acid-Base Balance
One of 3 pH regulation systems (with respiratory & buffering)
Conserves HCO₃⁻ and excretes metabolic acids
Regenerates bicarbonate ions
Excretes acid via:
• Binding to ammonia → NH₄⁺
• Reaction with monohydrogen phosphate (HPO₄²⁻)
Endocrine Function of the Kidney
Acts as both a hormone producer (primary) and target (secondary)
Synthesizes key hormones:
• Renin – blood pressure regulation
• Erythropoietin – RBC production
• 1,25-dihydroxyvitamin D – calcium/phosphate metabolism
• Prostaglandins – local blood flow and inflammation modulation
Renin
RAAS Initiator
Initial component of RAAS
Synthesized by renal medulla
Catalyzes conversion of angiotensinogen to angiotensin I
Erythropoietin
RBC Production Hormone
Acts on erythroid progenitor cells in bone marrow
Stimulates erythropoiesis
↓ in chronic renal insufficiency → anemia
1,25-Dihydroxyvitamin D – Active Vitamin D
Formed in kidney
Regulates Ca²⁺ & phosphate homeostasis
Promotes bone calcification
Deficiency in renal disease → osteomalacia
Prostaglandins
Renal Vasodilators
produced by kidneys (local effect)
↑ renal blood flow, Na⁺/H₂O excretion, renin release
Counteracts vasoconstriction from angiotensin & norepinephrine
Group of potent cyclic fatty acids
Creatinine Clearance – GFR Estimation Tool
Ideal for measuring clearance: freely filtered, not reabsorbed
Produced at constant rate (muscle metabolism)
Excreted solely by glomerular filtration
Directly correlates with muscle mass
Calculation compares 24-hr urine & serum creatinine to estimate GFR
Know the formula for the test

Estimated GFR – Clinical Use & Advantages
Recommended with every serum creatinine by NKF
Uses creatinine + age, sex, body size, race for estimation
No urine collection required
More routinely performed than CrCl
Detects chronic kidney disease (CKD) earlier
Evolution of eGFR Estimation Formulas
Cockcroft-Gault: Early formula; no BSA correction; assumes ↓CrCl in women
MDRD: Adds BSA correction; no weight input; uses age, sex, race, serum Cr
Underestimates in healthy, overestimates in underweight
CKD-EPI (2009): Less weight bias, more accurate; still uses race
CKD-EPI 2021 and Modern eGFR Estimation
Updated CKD-EPI equation (2021)
Uses creatinine, age, sex
Optionally includes Cystatin C
Eliminates race as a variable
Cystatin C in Renal Assessment
Low molecular weight protein; produced at steady rate by most tissues
Freely filtered by glomerulus, reabsorbed & catabolized in proximal tubule
Rises earlier than creatinine in acute kidney injury (AKI)
Detects ↓GFR before creatinine changes
Measured via immunoassay methods
Creatinine vs. Cystatin C in Monitoring Renal Function
Biologic variation: fluctuation around a homeostatic point
Creatinine:
Greater variation between individuals than within a single person
Better for long-term renal monitoring
Cystatin C:
Less variation between subjects
More sensitive to minor renal impairment
β₂-Microglobulin and Renal Function
Small, non-glycosylated peptide on most cell surfaces
Shed into plasma at a constant rate
Freely filtered by glomerulus; 99.9% reabsorbed by proximal tubules
Elevated serum: ↑ cell turnover (e.g., myeloproliferative, lymphoproliferative disorders, inflammation, renal failure)
Used in blood & urine to assess kidney damage, distinguish glomerular vs tubular disorders
Transplant monitoring: elevated levels may signal renal rejection
Myoglobin and Renal Implications
Type: Low molecular weight protein
Role: Binds and transports O₂ from plasma membrane to mitochondria of muscle cells
Toxicity: Nephrotoxin
Clinical association:
Released during acute skeletal or cardiac muscle injury
Rhabdomyolysis: massive myoglobin release → proximal tubule overload → acute renal failure (high Hgb, low RBC on dipstick)
Diagnostics: Early detection critical; measured by immunoassays
Albuminuria
Aka microalbuminuria—*presence of albumin in urine*
Early marker of diabetic nephropathy
Due to glomerulosclerosis → ↑ glomerular permeability
30–300 mg/day in 24-hr collection diagnostic
Random urine w/ creatinine: ACR >30 mg/g = albuminuria
Early detection allows for prevention via BP & glucose control
NGAL in AKI Detection
Neutrophil Gelatinase-Associated Lipocalin (NGAL)
25-kDa protein from neutrophils and epithelial cells (esp. proximal tubule)
Detectable in plasma/urine within 2–6 hrs of AKI onset
May elevate in systemic stress (non-AKI)
Research use only
NephroCheck for AKI Risk
FDA-cleared test
Identifies risk of moderate–severe AKI in critically ill patients
Predictive window: next 12 hours
AKI = acute kidney Injury
Specimen Collection (Urinalysis)
First morning urine preferred — more concentrated (esp. for protein)
Clean-catch midstream or catheterization
Stable: 1 hr RT or 8 hrs refrigerated (2–8 °C)
Improper storage → bacterial overgrowth
→ false + nitrite
→ urease activity → urea → ammonia → ↑ pH
→ CO₂ loss → ↑ pH
→ cast degradation, RBC lysis
Urine Color Findings
Correlates with concentration
Yellow/amber = urochromes
Yellow-brown to green = bile pigment oxidation
Red/brown after standing = porphyrins
Red/brown fresh = hemoglobin or RBCs
Brownish black after standing = alkaptonuria
Drugs/foods may alter color (beets, asparagus, food dyes)
Urine Odor Characteristics
Minimal clinical significance
Foul = UTI
Sweet/fruity = diabetes mellitus
Maple syrup odor = maple syrup urine disease
Urine Clarity Parameters
Cloudiness depends on pH and dissolved solids
May be affected by particulates
Urine Volume Significance
Normal range: 750–2000 mL/24 hrs (avg ~1.5 L/day)
Polyuria: diabetes mellitus or diabetes insipidus
Oliguria/Anuria (<200 mL/day): nephritis, obstruction, AKI, renal failure
Specific Gravity (SG)
Measures density of dissolved solids
Reflects hydration status or kidney concentrating ability
Method: Refractive index
Normal range: 1.003–1.035
Low SG: diabetes insipidus, pyelonephritis, glomerulonephritis
High SG: diabetes mellitus, CHF, dehydration, liver disease, adrenal insufficiency, nephrosis
Urine pH Overview
Perform on fresh specimen
Alkalinizes on standing
Normal range: 4.7–7.8
Acidity = phosphates
Alkalinity = Na⁺/H⁺ exchange (retains Na⁺ → ↑pH)
Alkaline causes: gastric HCl in duodenum, alkaline foods/meds, UTIs, contamination, Fanconi syndrome
Urine Glucose Testing
Normal result: Negative
Glucosuria occurs when renal threshold exceeds 180 mg/dL
Urine Ketone Testing
Hallmark of diabetes mellitus
Ketonuria may indicate ketoacidosis
Urine Protein Testing
Detects primarily albumin
May show false positives with alkaline or highly buffered urine
Confirm with more specific assays
Evaluate for casts as supportive evidence
Urine Nitrite Testing
Semi-quantitative for urinary nitrate reduction
Positive: suggests presence of nitrite-reducing bacteria (gram-negatives)
Negative: doesn’t rule out infection (e.g., gram-positive organisms)
Bilirubin in Urine
Product of hemoglobin degradation
Converted to urobilinogen in the gut
Presence may indicate liver dysfunction or biliary obstruction
Urobilinogen in Urine
Normally present in trace amounts
Reported as normal (not negative)
Elevated in liver disease or hemolysis
Blood in Urine
Positive with intact or lysed RBCs
Seen in:
Renal trauma
Infections
Obstruction (e.g. stones, tumors)
May also detect myoglobin
RBCs in Urine
2 RBCs/hpf = abnormal
Possible causes: trauma, vascular injury, calculi, pyelonephritis, cystitis
Contamination: menstrual blood, post-exercise
Hematuria + WBCs suggests infection
WBCs in Urine
5 WBCs/hpf = abnormal
Typically neutrophils
Seen in: glomerulonephritis, UTI, inflammation
In dilute urine → can appear enlarged & sparkle (glitter cells), no pathologic significance
Epithelial Cells in Urine
Types: squamous, renal, transitional
Squamous: common contaminant
Renal: associated with tubular injury
Transitional: from bladder/ureter lining
Miscellaneous Elements in Urine
Spermatozoa: age/gender dependent
Yeast: small, oval, budding
Trichomonas (STI): motile flagellated parasites
Bacteria in Urine
Normal urine = sterile
<20 orgs/hpf = possible contamination
20 orgs/hpf = clinically significant
Most are Gram-neg rods
Asymptomatic bacteriuria in high-risk groups can lead to pyelonephritis if untreated
Hyaline Casts
Composed of Tamm-Horsfall protein
Clear, gelatinous matrix
Often normal in small numbers
↑ in glomerular protein leakage
Phase contrast helps ID
Granular Casts
Coarse or finely granular texture
Common in chronic lead toxicity, pyelonephritis
Occasional presence = not necessarily pathologic
Cellular Casts
RBC Casts: glomerulonephritis
WBC Casts: nephron inflammation (e.g., pyelonephritis)
Epithelial Casts: can be normal occasionally
Waxy Casts: always pathologic, late stage renal disease
Fatty Casts: lipiduria (e.g., nephrotic syndrome)
Broad Casts: severe stasis, end-stage renal failure
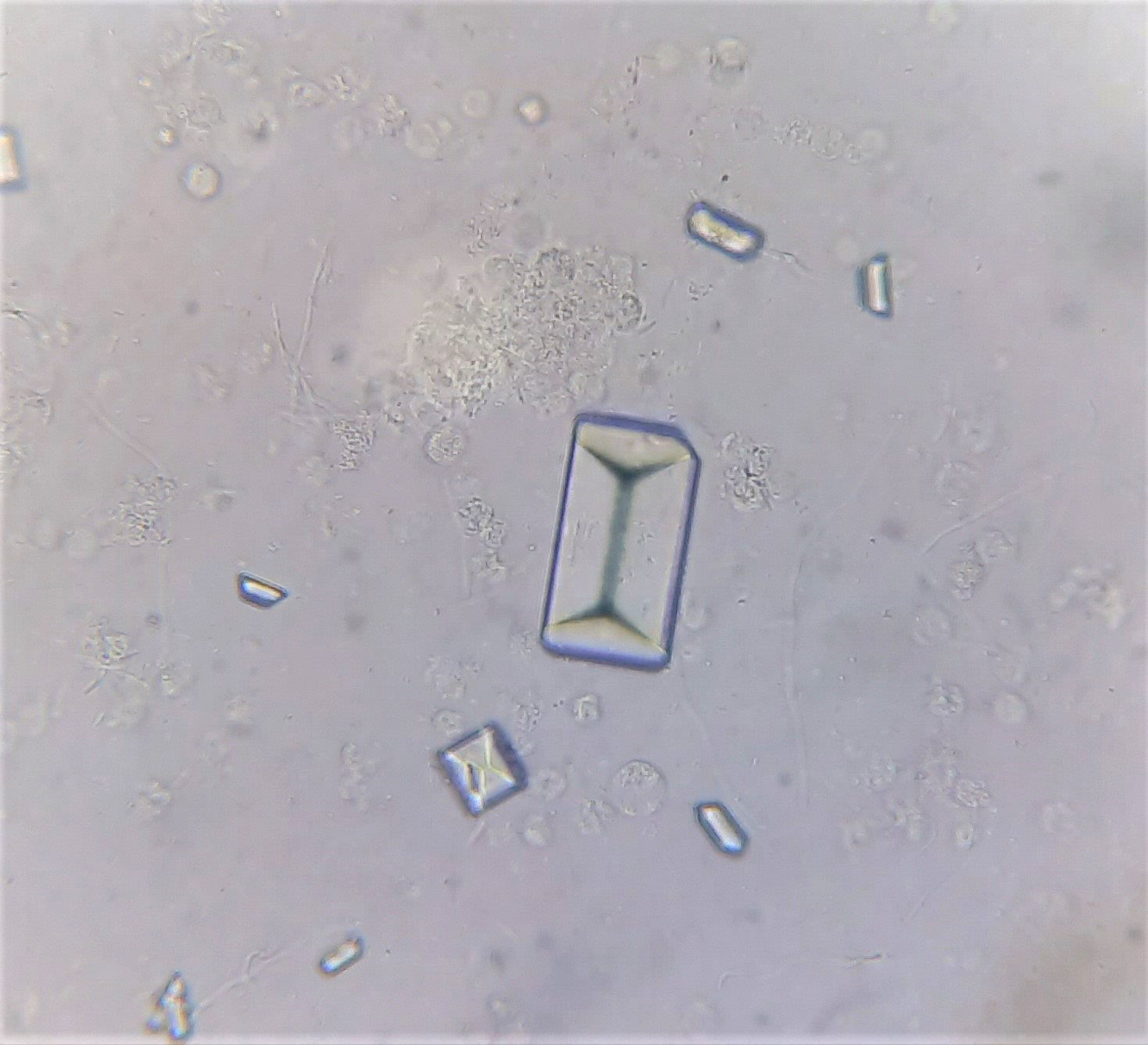
Crystals
Acidic Urine
Calcium oxalate
Amorphous urates
Uric acid
Cholesterol: nephrotic syndrome
Cystine: pathologic, seen in cystinuria/homocystinuria
Crystals
Alkaline Urine
Amorphous phosphates
Calcium carbonate
Triple phosphate (struvite) pictured
Ammonium biurate
Pathologic Crystals
Leucine
Tyrosine
Cystine
Sulfonamides
Cholesterol
Glomerular Damage Progression
Glomerular damage may initially preserve normal function
As disease progresses → tubular involvement ensues
Acute Glomerulonephritis
Glomerular lesions → ↓ capillary lumen, inflammation
Rapid onset: hematuria + proteinuria
Lab: ↓ GFR, anemia, ↑ BUN & creatinine, oliguria, Na⁺/H₂O retention, CHF
Hyaline, granular, and especially RBC casts
Commonly post-β-hemolytic strep
Other causes: drugs, AKI, immune complex disease
Chronic Glomerulonephritis
Prolonged inflammation → nephron scarring/loss
Often subclinical early
Slight ↓ renal function, mild proteinuria & hematuria
Nephrotic Syndrome Overview
Caused by glomerular basement membrane injury → ↑ permeability
Key findings:
• Massive proteinuria → hypoalbuminemia
• Generalized edema
• Hyperlipidemia + lipiduria (oval fat bodies)
Nephrotic Syndrome Pathophysiology
↑ Glomerular permeability → heavy proteinuria
Proteinuria results in:
• Hypoalbuminemia → edema, ↑ lipoprotein synthesis → hyperlipidemia
• Hypogammaglobulinemia → ↑ infection susceptibility
• Loss of antithrombin III → hypercoagulability
Tubular Disease Progression
Tubular defects arise as GFR declines in renal disease
Tubular dysfunction may become the dominant issue
Leads to:
↓ Excretion or reabsorption of substances
↓ Urinary concentrating ability
Renal Tubular Acidosis (RTA): Types
Clinically important tubular disorder affecting acid-base balance
Distal RTA: tubules can't maintain pH gradient between blood and tubular fluid
Proximal RTA: ↓ Bicarbonate reabsorption → hyperchloremic acidosis
RTA: General Findings
Rental Tubular Acidosis
↓ Serum phosphorus and uric acid
Glucose and amino acids in urine
Mild proteinuria possible
Interstitial nephritis may cause:
↓ GFR, ↓ urine concentration, ↓ acid excretion
WBC casts in urine
Na⁺ imbalance
Infection
Pyelonephritis (kidneys) vs Cystitis (bladder)
Diagnostic if >10⁵ colonies/mL
Bacteriuria = nitrite+, hematuria, pyuria
WBC casts = diagnostic for pyelonephritis
Obstruction
Raises intratubular pressure, causes nephron necrosis
Predisposes to infections
Upper: obstructed collecting duct
Lower: residual urine, slow voiding
Causes: tumors, congenital/acquired disease
↓ GFR, ↓ concentrating ability, ↓ acid excretion
Dx: urinalysis, culture, BUN/Cr, CBC, imaging
Renal Calculi Overview
Kidney stones = crystallized substance aggregates
Calcium oxalate = most common type
Caused by ↓ urine flow rate + ↑ insoluble substances in urine
Symptoms:
• Hematuria
• UTIs
• Abdominal pain (characteristic)
Acute Kidney Injury (AKI)
Sudden drop in renal function from toxic or hypoxic insult
50% of Pts admitted to ICU
Diagnosed using:
• Serum creatinine
• Urinary output
Prerenal AKI
Blood supply defect before reaching kidneys
Caused by cardiovascular failure → hypovolemia
Intrinsic AKI
Defect occurs within the kidney
Most common: acute tubular necrosis
Also caused by glomerulonephritis or vascular obstruction/inflammation
Postrenal AKI
Defect in urinary tract after the kidney
Caused by lower urinary obstruction or bladder ruptureRenal Hypertension
Renal Hypertension
Caused by decreased renal perfusion (trauma, artery/arteriole narrowing)
Chronic ischemia → nephron dysfunction & necrosis
Triggers RAAS activation → vasoconstriction → persistent hypertension
Lab findings: ↑ aldosterone, ↑ serum Na, ↓ serum K, ↑ urine K
Acute Kidney Injury: Dialysis Therapy
Indications: uremic symptoms, hyperkalemia, acidosis
Only options in irreversible failure:
• Traditional dialysis
• Peritoneal dialysisMonitoring via lab tests required
Most effective dialysis = 10–12% of normal kidney function
Associated with physical disability and reduced quality of life
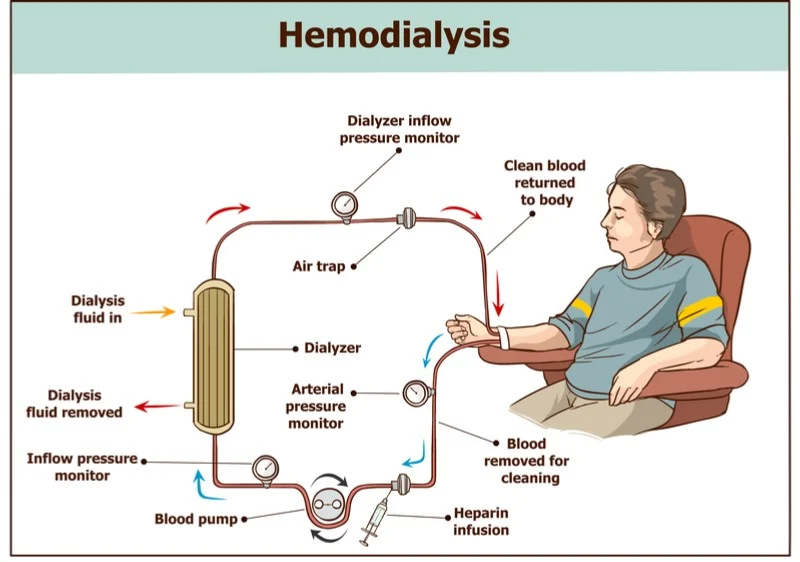
Hemodialysis
Synthetic membrane outside the body
Arterial blood pumped through dialysate at high speed
Blood returned to venous circulation; dialysate discarded
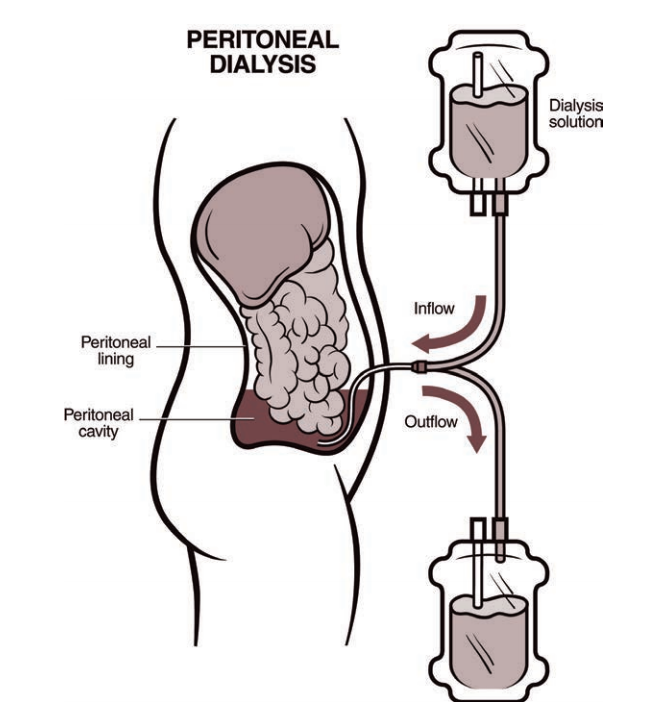
Peritoneal Dialysis (CAPD)
Peritoneal wall serves as dialysate membrane
Less efficient: small solutes (e.g. K⁺) cleared slowly
Steady clearance of larger solutes over time
Hemofiltration
Continuous venovenous or arteriovenous systems
Combines hemofiltration + dialysis for slow, sustained clearance
Used in intensive care (continuous renal replacement therapy)
Kidney Transplantation
Offers best chance for full recovery
Limited donor supply → wait times range months to years
Donor source: 80% cadaver, 20% live (live grafts = better outcomes)
Requires ABO & HLA matching + lifelong immune suppression
Grafts can last decades (mean half-life cadaver: 7 yrs)
3-yr survival: 65–85% (higher with live donor)
Survival similar across CAPD, hemodialysis, cadaver transplant
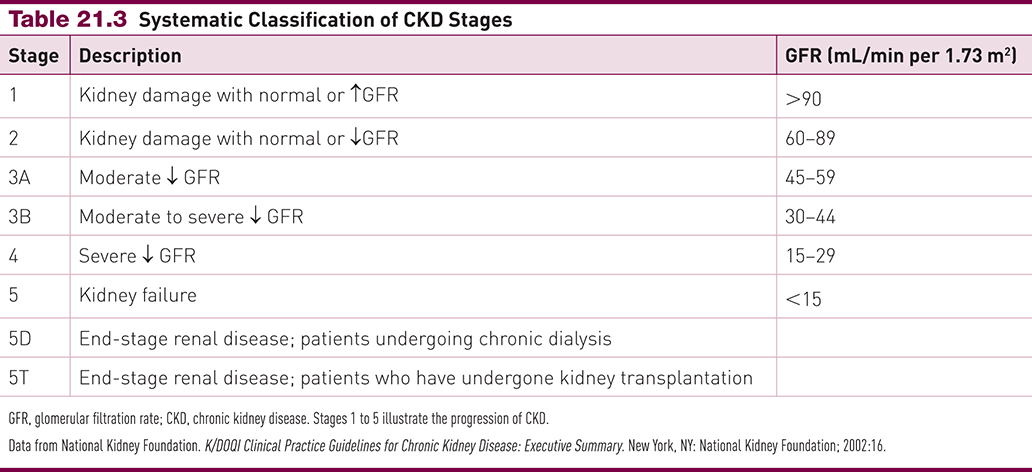
Chronic Renal Failure (CKD)
Incidence rising due to diabetes, aging, obesity, metabolic syndrome
Diabetes: leading cause (→ 45% of kidney failure cases)
Leads to glucosuria, polyuria, nocturia
Diuresis of hyperosmotic urine → strain on nephrons
Mild proteinuria, hypertension common
Metabolic syndrome (≥3: abd. obesity, HTN, low HDL, hypertriglyceridemia, hyperglycemia)
→ 2.6× increased CKD risk
Know the levels of CKD for EXAM
monitor Delta changes. they don’t get better