Module 2 – Foundations In Chemistry
0.0(0)
0.0(0)
Card Sorting
1/125
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
126 Terms
1
New cards
Aluminium will combine directly with fluorine.
Write the equation for the reaction between aluminium and fluorine.
Write the equation for the reaction between aluminium and fluorine.
2Al + 3F2 → 2AlF3
2
New cards
Describe what is meant by the term ionic lattice, in terms of the type and arrangement of particles present.
Repeating pattern of oppositely charged ions
3
New cards
Draw a ‘dot-and-cross ’ diagram for aluminium fluoride.
4
New cards
What is meant by the term covalent bond ?
A shared pair of electrons
5
New cards
Draw a ‘dot-and-cross ’ diagram to show the bonding in a boron tribromide molecule.
6
New cards
State whether the following substances conduct electricity when solid or molten, and explain your answers in terms of the particles involved:
• aluminum
• aluminum fluoride
• boron tribromide.
• aluminum
• aluminum fluoride
• boron tribromide.
* Aluminum conducts in solid and molten states
* Aluminum has delocalised electrons
* Aluminum fluoride conducts when molten AND because it has mobile ions
* Aluminum fluoride does not conduct when solid AND Solid aluminum fluoride has ions which are fixed in an (ionic) lattice
* Boron tribromide does not conduct in solid and molten states AND Boron tribromide has no mobile electrons
* Aluminum has delocalised electrons
* Aluminum fluoride conducts when molten AND because it has mobile ions
* Aluminum fluoride does not conduct when solid AND Solid aluminum fluoride has ions which are fixed in an (ionic) lattice
* Boron tribromide does not conduct in solid and molten states AND Boron tribromide has no mobile electrons
7
New cards
Aluminium has 13 successive ionisation energies.
(i) Write the equation for the third ionisation energy of aluminium.
(i) Write the equation for the third ionisation energy of aluminium.
Al2+(g) → Al3+(g) + e–
8
New cards
State how and explain why the attraction between nuclei and outermost electrons in gaseous atoms varies across Period 3.
The attraction (between nuclei and outermost electrons) increases (across the period) AND The nuclear charge increases
9
New cards
The table shows the boiling points of ammonia, fluorine and bromine. Explain the different boiling points of NH3, F2 and Br2
* NH3 has hydrogen bonding
* F2 AND Br2 have London forces
* the intermolecular force in Br2 is stronger than that of F2 Because bromine has more electrons than fluorine
* The London forces in Br2 are greater than hydrogen bonding in NH3 AND hydrogen bonding in NH3 is stronger than London forces in F2
* F2 AND Br2 have London forces
* the intermolecular force in Br2 is stronger than that of F2 Because bromine has more electrons than fluorine
* The London forces in Br2 are greater than hydrogen bonding in NH3 AND hydrogen bonding in NH3 is stronger than London forces in F2
10
New cards
Complete the diagram below to show hydrogen bonding between the H2O molecule shown and one other H2O molecule.
11
New cards
State and explain two anomalous properties of ice caused by hydrogen bonding.
Ice is less dense than water since the molecules in ice are held apart by hydrogen bonds
Ice has a relatively high melting point because hydrogen bonds are relatively strong
Ice has a relatively high melting point because hydrogen bonds are relatively strong
12
New cards
Draw a ‘dot-and-cross’ diagram to show the bonding in CO2.
13
New cards
Silicon dioxide, SiO2, has the same structure and bonding as diamond. State the structure and bonding in SiO2
Giant covalent (lattice)
14
New cards
Describe and explain the electrical conductivity of sodium oxide, Na2O, and sodium in their solid and molten states.
* Sodium conducts in the solid and molten states
* Sodium has delocalised electrons in both states
* Na2O conducts when molten and not when solid
* Molten Na2O has ions which are mobile
* Solid Na2O has ions which are fixed AND in an (ionic) lattice
* Sodium has delocalised electrons in both states
* Na2O conducts when molten and not when solid
* Molten Na2O has ions which are mobile
* Solid Na2O has ions which are fixed AND in an (ionic) lattice
15
New cards
Phosphine gas, PH3, can be prepared by adding phosphorus, P4, to warm concentrated aqueous sodium hydroxide as shown in the equation below.
P4(s) + 3NaOH(aq) + 3H2O(l) PH3(g) + 3NaH2PO2(aq)
Using oxidation numbers, explain why this is a disproportionation reaction
P4(s) + 3NaOH(aq) + 3H2O(l) PH3(g) + 3NaH2PO2(aq)
Using oxidation numbers, explain why this is a disproportionation reaction
P in P4 is 0 AND in PH3 is –3 AND in NaH2PO2 is (+)1
Phosphorus has been oxidised (from 0) to +1
Phosphorus has been reduced (from 0) to –3
Phosphorus has been oxidised (from 0) to +1
Phosphorus has been reduced (from 0) to –3
16
New cards
A chemist reacts 1.86 g of P4 with excess NaOH(aq). Calculate the volume of phosphine gas, in cm3, produced at room temperature and pressure, RTP
Correctly calculates amount of P4 = 1.86/124.0 = 0.015(0) mol Correctly calculates volume of PH3 = 0.015(0) x 24000 = 360 (cm3)
17
New cards
Phosphine gas burns in air to form an oxide of phosphorus, P4O10, and water. Write the equation for this reaction.
4PH3 + 8O2 → P4O10 + 6H2O
18
New cards
Phosphoric acid, H3PO4, can be made by reacting P4O10 with water.
Sodium phosphate, Na3PO4, is a salt that can be prepared by reacting H3PO4 with sodium hydroxide, NaOH.
A student prepared a solution of Na3PO4 by reacting 15.0 cm3 of 0.100 mol dm–3 H3PO4 with 0.200 mol dm–3 NaOH.
(i) Why is Na3PO4 described as a salt of H3PO4?
Sodium phosphate, Na3PO4, is a salt that can be prepared by reacting H3PO4 with sodium hydroxide, NaOH.
A student prepared a solution of Na3PO4 by reacting 15.0 cm3 of 0.100 mol dm–3 H3PO4 with 0.200 mol dm–3 NaOH.
(i) Why is Na3PO4 described as a salt of H3PO4?
The hydrogen ions
19
New cards
Calculate the amount, in moles, of H3PO4 in 15.0 cm3 of 0.100 mol dm–3 H3PO4
0\.0015
20
New cards
The equation for the preparation of Na3PO4 from NaOH and H3PO4 is shown below.
3NaOH(aq) + H3PO4(aq) Na3PO4(aq) + 3H2O(l)
Calculate the volume of 0.200 mol dm–3 NaOH that reacts exactly with 15.0 cm3 of 0.100 mol dm–3 H3PO4.
3NaOH(aq) + H3PO4(aq) Na3PO4(aq) + 3H2O(l)
Calculate the volume of 0.200 mol dm–3 NaOH that reacts exactly with 15.0 cm3 of 0.100 mol dm–3 H3PO4.
) 22.5
21
New cards
NH3 and PH3 are both simple molecules. The boiling points of NH3 and PH3 are shown in the table below.
hydrogen bonding
Permanent dipole
Permanent dipole
22
New cards
Suggest why PH3 has a lower boiling point than NH3.
the intermolecular forces are weaker in PH3
23
New cards
What is a dative covalent bond?
Both electrons have been donated by one atom
24
New cards
Draw a ‘dot-and-cross’ diagram to show the bonding in H3NBF3
25
New cards
) The F–B–F bond angle in BF3 is different from the F–B–F bond angle in H3NBF3. Complete the table to predict the F–B–F bond angles in BF3 and in H3NBF3
BF3 = 120(°)
H3NBF3 = 109.5(°)
H3NBF3 = 109.5(°)
26
New cards
Explain why the student might expect the H–N–H bond angle to be larger in H3NBF3 than in NH3.
NH3 has three bonding pairs and one lone pair of electrons
H3NBF3 has four bonding pairs (and no lone pairs) of electrons
Lone pair of electrons repels more than bonding pairs
H3NBF3 has four bonding pairs (and no lone pairs) of electrons
Lone pair of electrons repels more than bonding pairs
27
New cards
Bleach can be made by reacting chlorine with cold aqueous sodium hydroxide. A solution of bleach contains the chlorate compound NaClO. Write the equation for the reaction taking place
2NaOH + Cl2 → NaClO + NaCl + H2O
28
New cards
Give the systematic name for NaClO3.
Sodium chlorate(V)
29
New cards
When heated, NaClO3 disproportionate as shown in the equation below.
4NaClO3 3NaClO4 + NaCl
Using oxidation numbers, explain why this is a disproportionation reaction.
4NaClO3 3NaClO4 + NaCl
Using oxidation numbers, explain why this is a disproportionation reaction.
Cl in NaClO3 is (+)5 AND Cl in NaClO4 is (+)7 AND Cl in NaCl is –1
Chlorine has been both oxidised and reduced
Chlorine has been oxidised from (+)5 to (+)7 AND chlorine has been reduced from (+)5 to –1
Chlorine has been both oxidised and reduced
Chlorine has been oxidised from (+)5 to (+)7 AND chlorine has been reduced from (+)5 to –1
30
New cards
State one valid reason that supports the scientists’ case and state one reason why chlorine should be added to drinking water
Chlorine is toxic but it kills bacteria
31
New cards
Draw a ‘dot-and-cross’ diagram to show the bonding in a molecule of CH3Cl.
32
New cards
Name the shape of a molecule of CH3Cl .
Tetrahedral
33
New cards
Describe a simple chemical test that you could carry out to show that brine contains aqueous chloride ions. How would you confirm that no other halide ions are present?
Add silver nitrate
White precipate
Ag+ + Cl– → AgCl
Add dilute NH3 and precipitate (completely) dissolves
White precipate
Ag+ + Cl– → AgCl
Add dilute NH3 and precipitate (completely) dissolves
34
New cards
Explain, with the aid of a labelled diagram, what is meant by the term metallic bonding
metallic bond as (electrostatic) attraction between the electrons and the positive ions
35
New cards
Write the equation for the reaction of sodium with oxygen to form sodium oxide.
4 Na + O2 → 2 Na2O
36
New cards
State what is meant by the term ionic bond.
(electrostatic) attraction between oppositely charged ions
37
New cards
Draw a ‘dot-and-cross’ diagram to show the bonding in Na2O.
38
New cards
Compare and explain the electrical conductivities of sodium and sodium oxide in the solid and liquid states.
* sodium is a (good) conductor because it has mobile electrons
* sodium oxide does not conduct as a solid
* sodium oxide conducts when it is a liquid
* ions cannot move in a solid
* ions can move OR are mobile when liquid
* sodium oxide does not conduct as a solid
* sodium oxide conducts when it is a liquid
* ions cannot move in a solid
* ions can move OR are mobile when liquid
39
New cards
Explain why there is a difference in the melting points of K, KBr and H2O
* In K, (electrostatic attraction between) positive ions/cations AND e– / electron
* In KBr, (electrostatic attraction between) oppositely OR positively AND negatively charged ions
* K has metallic bonding while KBr has oppositely charged ions
* In H2O hydrogen bonding between molecules
* ionic bonding > metallic bonding > hydrogen bonding
* In KBr, (electrostatic attraction between) oppositely OR positively AND negatively charged ions
* K has metallic bonding while KBr has oppositely charged ions
* In H2O hydrogen bonding between molecules
* ionic bonding > metallic bonding > hydrogen bonding
40
New cards
2K(s) + 2H2O(l) 2KOH(aq) + H2(g)
0\.2346 g of potassium is reacted with excess water.
Calculate the volume of gas formed.
0\.2346 g of potassium is reacted with excess water.
Calculate the volume of gas formed.
amount of K = 0.2346 / 39.1 OR = 6.(00) × 10–3 OR 0.006(00) mol amount of H2 = (mol of K) / 2 OR = 3.(00) × 10–3 OR 0.003(00) mol
Volume of gas = (mol of H2) × 24000 OR = 72(.0)
Volume of gas = (mol of H2) × 24000 OR = 72(.0)
41
New cards
What is meant by the term electronegativity?
The ability of an atom to attract electrons in a covalent bond
42
New cards
Show, using δ+ and δ– symbols, the permanent dipoles on each of the following bonds.
δ+N–F δ- AND δ-N–Br δ+
43
New cards
State the shape of a molecule of SF6.
octahedral
44
New cards
Using outer electron shells only, draw ‘dot-and-cross’ diagrams for molecules of BF3 and NH3.
Use your diagrams to explain why a molecule of BF3 has bond angles of 120° and NH3 has bond angles of 107°.
Use your diagrams to explain why a molecule of BF3 has bond angles of 120° and NH3 has bond angles of 107°.
electron pairs repel
NH3 has one lone pair and three bonding pairs of electrons AND lone pair of electrons repels more than bonding pairs
BF3 has three (bonding) pairs of electrons (which repel equally)
NH3 has one lone pair and three bonding pairs of electrons AND lone pair of electrons repels more than bonding pairs
BF3 has three (bonding) pairs of electrons (which repel equally)
45
New cards
Molecules of BF3 contain polar bonds, but the molecules are non-polar. Suggest an explanation for this difference.
BF3 is symmetrical
The dipoles cancel out
The dipoles cancel out
46
New cards
Draw a ‘dot-and-cross ’ diagram to show the bonding in magnesium sulfide. Show outer electron shells only.
47
New cards
Draw a ‘dot-and-cross ’ diagram of a molecule of F2O. Show outer electron shells only
48
New cards
Predict the bond angle in an F2O molecule. Explain your answer.
* Predicted bond angle 104–105O.
* There are 2 bonded pairs and 2 lone pairs
* Lone pairs repel more than bonded pairs
* There are 2 bonded pairs and 2 lone pairs
* Lone pairs repel more than bonded pairs
49
New cards
Draw a labelled diagram to show hydrogen bonding between two molecules of liquid ammonia.
50
New cards
Describe and explain one anomalous property of water which results from hydrogen bonding.
H2O has a relatively high boiling point since a lot of energy is needed to overcome hydrogen bonds
51
New cards
Explain, using your ‘dot-and-cross’ diagram, why ammonia has this shape and has a bond angle of 107°.
* pyramidal
* There are 3 bonded pairs and 1 lone pair
* Lone pairs repel more than bonded pairs
* There are 3 bonded pairs and 1 lone pair
* Lone pairs repel more than bonded pairs
52
New cards
Complete the electron configuration of the Cl – ion.
1s2 2s2 2p6 3s2 3p6
53
New cards
State the shape of, and bond angle in, an NH4 + ion.
* tetrahedral
* 109.5º
* 109.5º
54
New cards
Draw a ‘dot-and-cross’ diagram to show the bonding in NH4 +
Draw a ‘dot-and-cross’ diagram to show the bonding in NH4
\+.
.
Draw a ‘dot-and-cross’ diagram to show the bonding in NH4
\+.
.
\
\
\
55
New cards
She noticed that when the ammonium chloride was solid it did not conduct electricity. However, when ammonium chloride was dissolved in water, the resulting solution did conduct electricity. Explain these observations.
ions OR electrons cannot move in a solid but can move in solution
56
New cards
Write a balanced equation to show how ammonium sulfate could be formed by the reaction between aqueous ammonia and sulfuric acid.
2NH3 + H2SO4 → (NH4)2SO4
57
New cards
Explain what is meant by the term salt.
when the H+ in an acid is replaced by a metal ion
58
New cards
Why is ammonia acting as a base in the neutralisation of ammonia and sulfuric acid?
It accepts a protons
59
New cards
What is the relative formula mass of (NH4 ) 2SO4? Give your answer to one decimal place.
132\.1
60
New cards
MnO2(s) + 4HCl(aq) MnCl 2(aq) + 2H2O(l) + Cl 2(g)
Using oxidation numbers, show what has been oxidised and what has been reduced in this reaction.
Using oxidation numbers, show what has been oxidised and what has been reduced in this reaction.
Cl (has been oxidised) from Cl = –1 to Cl = 0
Mn (has been reduced) from Mn = +4 to Mn = +2
Mn (has been reduced) from Mn = +4 to Mn = +2
61
New cards
Complete the electron configuration of a manganese atom.
1s2 2s2 2p6 3s2 3p6 3d5 4s2
62
New cards
Chlorine gas can be added to a cold, dilute alkaline solution to form bleach. Write the equation for this reaction.
Cl2 + 2NaOH → NaClO + NaCl + H2O
63
New cards
A student bubbles chlorine gas through aqueous potassium iodide. A reaction takes place. (i) State what the student would observe.
(The solution would turn) yellow OR orange OR brown
64
New cards
Explain what is meant by the term electronegativity
The ability of an atom to attract electrons in a (covalent) bond
65
New cards
Draw a 3-D diagram of a molecule of CH2Cl 2. Use partial charges to indicate polar bonds.
66
New cards
Explain why a CH2Cl 2 molecule is polar
The dipoles do not cancel out OR Because the molecule is non-symmetrical
67
New cards
Bromine has two isotopes, Br–79 and Br–81. The relative atomic mass of bromine is 79.9. Calculate the percentage of Br–79 atoms in a sample of bromine.
55%
68
New cards
What is meant by the term isotopes?
Atom(s) of an element with different numbers of neutrons
69
New cards
Different isotopes of antimony have the same chemical properties. Explain why.
same number of electrons in outer shell OR same electron configuration OR electron structure
70
New cards
Define the term relative atomic mass.
The (weighted) mean mass of an atom compared with 1/12th (the mass) of (one atom of) carbon-12
71
New cards
Predict the shape of a molecule of SbCl 3. Explain your answer.
(Trigonal) Pyramidal
(Sb has) three bonding pairs AND one lone pair of electrons
Pairs of electrons repel
(Sb has) three bonding pairs AND one lone pair of electrons
Pairs of electrons repel
72
New cards
SbCl 3 molecules are polar. Explain why
There is a difference in electronegativities (between Sb and Cl) OR (Sb-Cl) bonds are polar OR have a dipole OR Dipoles seen on the diagram
The molecule is not symmetrical AND dipoles do not cancel
The molecule is not symmetrical AND dipoles do not cancel
73
New cards
State and explain the trend in atomic radius from Li to F.
atomic radius decreases AND nuclear charge increases or number of protons increases
electrons are in same shell OR (outer) electrons experience similar or same shielding OR same number of shells
Greater nuclear attraction on (outer) electrons or shells
electrons are in same shell OR (outer) electrons experience similar or same shielding OR same number of shells
Greater nuclear attraction on (outer) electrons or shells
74
New cards
Complete the electron configuration of a bromide ion.
(1s2) 2s2 2p6 3s2 3p6 3d10 4s2 4p6
75
New cards
A student adds a small volume of aqueous silver nitrate to an aqueous solution of bromide ions in a test-tube. The student then adds a similar volume of dilute aqueous ammonia to the same test-tube. Describe what the student would see in the test-tube after the addition of aqueous ammonia.
Cream AND precipitate
76
New cards
Chlorine reacts with aqueous sodium hydroxide to form bleach. Write the equation and state the conditions for this reaction.
Equation: 2NaOH + Cl2 NaCl + NaClO + H2O
Conditions :cold AND dilute (sodium hydroxide)
Conditions :cold AND dilute (sodium hydroxide)
77
New cards
A disproportionation reaction takes place as shown below.
3Cl 2(g) + 6NaOH(aq) 5NaCl (aq) + NaCl O3(aq) + 3H2O(
State what is meant by disproportionation and show that disproportionation has taken place in this reaction. Use oxidation numbers in your answer.
3Cl 2(g) + 6NaOH(aq) 5NaCl (aq) + NaCl O3(aq) + 3H2O(
State what is meant by disproportionation and show that disproportionation has taken place in this reaction. Use oxidation numbers in your answer.
* (Disproportionation) is the (simultaneous) oxidation and reduction of the same element (in the same redox reaction)
* Cl in Cl2 is 0 AND Cl in NaCl is -1 AND Cl in NaClO3 is +5
* Chlorine has been oxidised from 0 to +5 AND Chlorine has been reduced from 0 to –1
* Cl in Cl2 is 0 AND Cl in NaCl is -1 AND Cl in NaClO3 is +5
* Chlorine has been oxidised from 0 to +5 AND Chlorine has been reduced from 0 to –1
78
New cards
Write the formula of cerium(III) sulfate and, explain what has happened to the cerium in this reaction in terms of the number of electrons transferred
Ce2(SO4)3
(Cerium) loses three electrons
(Cerium) loses three electrons
79
New cards
How has a salt been formed in this reaction?
A hydrogen ion (of an acid) has been replaced by a metal ion
80
New cards
Europium, atomic number 63, reacts with oxygen at room temperature.
4Eu + 3O2 2Eu2O3
Calculate the volume of oxygen, in cm3, required to fully react with 9.12 g of europium at room temperature and pressure.
4Eu + 3O2 2Eu2O3
Calculate the volume of oxygen, in cm3, required to fully react with 9.12 g of europium at room temperature and pressure.
Amount of Eu = 9.12/ 152.0 = 0.06(00) mol
Amount of O2 = 0.0600 x 3/4 = 0.045(0) mol and Volume of O2 = 0.0450 x 24000 = 1080 cm
Amount of O2 = 0.0600 x 3/4 = 0.045(0) mol and Volume of O2 = 0.0450 x 24000 = 1080 cm
81
New cards
State what is meant by the term empirical formula
The simplest whole number ratio of atoms (of each element) present in a compound
82
New cards
Ytterbium, atomic number 70, is the first element in the Periodic Table to have the first four shells full.
(i) State the number of electrons in the fourth shell of ytterbium.
(ii) How many orbitals are there in the third shell of ytterbium?
(i) State the number of electrons in the fourth shell of ytterbium.
(ii) How many orbitals are there in the third shell of ytterbium?
i) 32
ii) 9
ii) 9
83
New cards
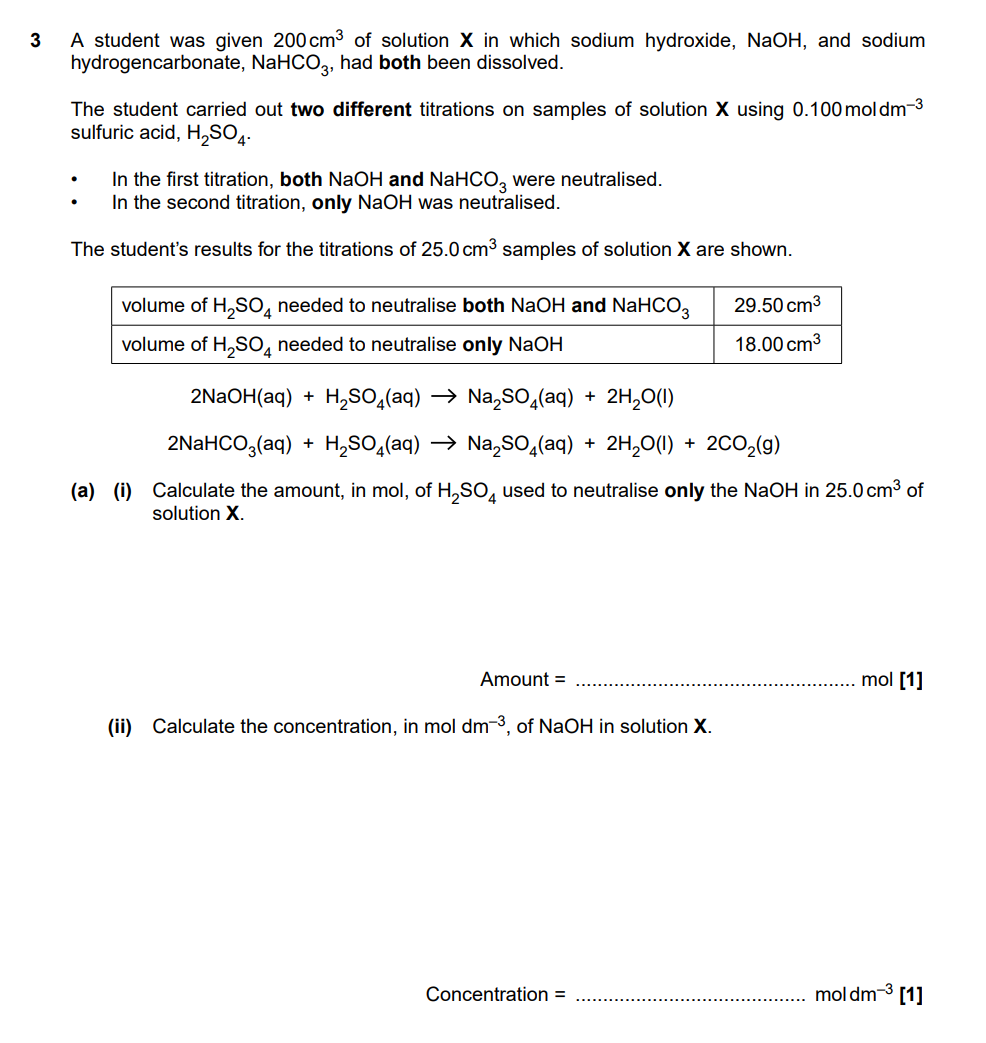
(i) Mol of H2SO4 = 0.100 x 18.00/1000 = 1.80 x 10–3 mol
(ii) Mol of NaOH in = 1.80 x 10–3 x 2 x 1000/25.0 = 0.144 mol dm–3
(ii) Mol of NaOH in = 1.80 x 10–3 x 2 x 1000/25.0 = 0.144 mol dm–3
84
New cards
Explain what is meant by the terms base and alkali.
Base: A substance which readily accepts H+ ions (from an acid) Alkali: releases OH– ions into (aqueous) solution
85
New cards
) A student prepared some calcium hydroxide by adding a small piece of calcium to a large excess of water. Describe what the student would observe and write the equation for the reaction.
Fizzing and the metal would dissolve
Ca + 2H2O → Ca(OH)2 + H2
Ca + 2H2O → Ca(OH)2 + H2
86
New cards
A student prepares a solution of calcium nitrate from calcium carbonate. What reagent would the student need to use? Write the equation for the reaction.
Nitric acid OR HNO3
CaCO3 + 2HNO3 → Ca(NO3)2 + H2O + CO2
CaCO3 + 2HNO3 → Ca(NO3)2 + H2O + CO2
87
New cards
A compound used as a fertiliser has the following composition by mass: C, 20.00%; H, 6.67%; N, 46.67%; O, 26.66%. Calculate the empirical formula of this compound.
CH4N2O
88
New cards
A salt used as a fertiliser has the empirical formula H4N2O3. Suggest the formulae of the ions present in this salt.
NH4+
NO3
NO3
89
New cards
Calcium phosphate(V), Ca3(PO4) 2, is another salt used in fertilisers. Calcium phosphate(V) can be prepared by reacting together an acid and a base.
(i) Suggest the formula of the acid used to prepare Ca3(PO4) 2.
(ii) Name a base which could be used to prepare Ca3(PO4) 2.
(i) Suggest the formula of the acid used to prepare Ca3(PO4) 2.
(ii) Name a base which could be used to prepare Ca3(PO4) 2.
H3PO4
Calcium oxide OR calcium hydroxide OR calcium carbonate
Calcium oxide OR calcium hydroxide OR calcium carbonate
90
New cards
) Silicon reacts with chlorine to form molecules of silicon tetrachloride, SiCl 4. How many molecules are present in 8.505 g of SiCl 4?
170\.1 (ALLOW in working shown as 28.1 + 35.5 x 4) Correctly calculates amount of molecules 8.505 / 170.1 = 0.05(00) mol Correctly calculates number of molecules 0.05 x 6.02 x 1023 = 3.01 x 1022
91
New cards
Ions of two isotopes of iron are
53Fe2+ 56Fe2+
Which statement is correct?
A The ions of both the isotopes have the electronic configuration 1s22s22p63s23p64s23d6
B The ions of both the isotopes contains 26 neutrons
C 53Fe2+ has fewer protons than 56Fe2+
D After acceleration to the same kinetic energy 56Fe2+ will move more slowly than 53Fe2
53Fe2+ 56Fe2+
Which statement is correct?
A The ions of both the isotopes have the electronic configuration 1s22s22p63s23p64s23d6
B The ions of both the isotopes contains 26 neutrons
C 53Fe2+ has fewer protons than 56Fe2+
D After acceleration to the same kinetic energy 56Fe2+ will move more slowly than 53Fe2
D After acceleration to the same kinetic energy 56Fe2+ will move more slowly than 53Fe2
92
New cards
Deduce the formula of the compound that contains 2+ ions and 3− ions that both have the same electron configuration as argon.
Ca3P2
93
New cards
Deduce the formula of the ion that has a charge of 2+ with the same electron configuration as krypton.
Sr2+
94
New cards
State and explain the general trend in first ionisation energy across Period 3.
* General increase
* Greater nuclear charge / more protons
* Same shielding / electrons added to same shell
* Stronger attraction (from nucleus) for outer electron(s)
* Greater nuclear charge / more protons
* Same shielding / electrons added to same shell
* Stronger attraction (from nucleus) for outer electron(s)
95
New cards
Give one example of an element which deviates from the general trend in first ionisation energy across Period 3. Explain why this deviation occurs
* Aluminium / Al (lower than Mg) CE if not Al or S
* (Outer) electron in (3)p orbital / sub-shell (level)
* (3p) higher in energy
* Sulfur / S (lower than P) (Outer) electrons in (3)p orbital begin to pair Repel
* (Outer) electron in (3)p orbital / sub-shell (level)
* (3p) higher in energy
* Sulfur / S (lower than P) (Outer) electrons in (3)p orbital begin to pair Repel
96
New cards
Identify the Period 3 element that has the highest melting point. Explain your answer by reference to structure and bonding
* Silicon
* Giant covalent structure / macromolecule
* Covalent (bonds) Giant covalent scores
* Many / strong (covalent bonds) or (covalent bonds) need lots of energy to break
* Giant covalent structure / macromolecule
* Covalent (bonds) Giant covalent scores
* Many / strong (covalent bonds) or (covalent bonds) need lots of energy to break
97
New cards
Use the Periodic Table to deduce the full electron configuration of calcium.
1s2 2s2 2p6 3s2 3p6 4s2
98
New cards
Write an ionic equation, with state symbols, to show the reaction of calcium with an excess of water.
Ca(s)+ 2H2O(l) → Ca2+(aq) + 2OH– (aq) + H2(g)
99
New cards
State the role of water in the reaction with calcium.
Oxidising agent
100
New cards
Write an equation to show the process that occurs when the first ionisation energy of calcium is measured.
Ca(g) → Ca+ (g) + e–