1. Atomic Theory
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
What does Dalton’s model say all matter is made of?
Atoms
What 3 things does Dalton’s Model say that atoms are?
Indivisible
indestructible
Identical in mass and properties
What does Dalton’s model say that compounds are formed by?
A combination of 2 or more different kinds of atoms
What does Dalton’s Model say a chemical reaction is?
A rearrangement of atoms
What does the Thomson’s Model say atoms are?
Uniform spheres of positively charged matter in which electrons are embedded
What does Rutherford’s Model say about the size of negatively charged electrons?
They’re small
What does the Rutherford’s Model and Bohr’s Model say that negatively charged electrons do?
Orbit around the nucleus
Where does Bohr’s Model say that the lowest energy is found?
In the smallest orbit
What does Bohr’s Model say the energy of the orbit is related to?
Its size
When does Bohr’s Model say radiation is absorbed or emitted?
When an electron moves from one orbit to another
What is the wavelength of a moving body inversely proportional to?
Its momentum
How do you calculate momentum?
Mass (kg) x velocity (m/s)
What is the motion of large bodies better described by?
Classical mechanics
What is the motion of particles better described by?
Quantum mechanics
Long wavelength has?
Small frequency and energy
Short wavelength has?
High frequency and energy
What is the principle quantum number(n)?
Number of period or row of the periodic table
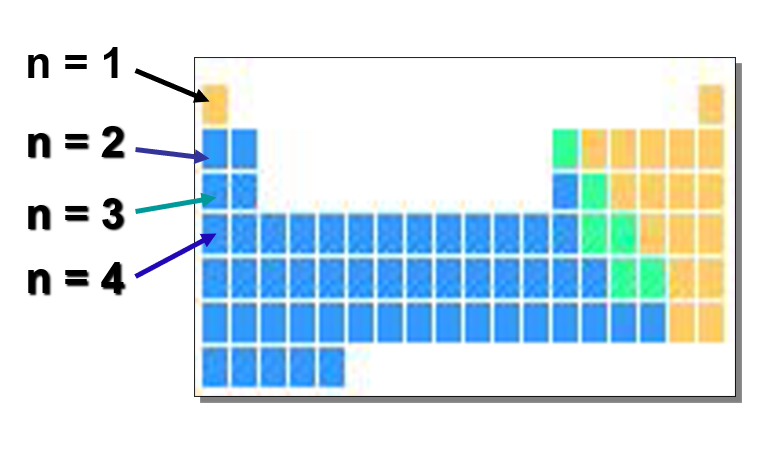
What is the angular quantum number(l)?
(n - 1) e.g.
if n=2
l=1
What is the magnetic quantum number(ml)?
if l=1
ml = -1,0,1
What is ms spin quantum number?
either -1/2 or +1/2 doesn’t matter
What does the quantum number n determine?
Size
What does the quantum number l determine?
Shape
What does the quantum number ml determine?
Orientation
What is shape of 1s orbital?
no nodes
What is shape of 2s orbital?
1 node
What is shape of 3s orbital?
2 nodes
What is the shape of 2p orbitals?
Dumbell with an angular node passing through the nucleus
What is the shape of 3d orbitals?
Four leaf clover shaped with 2 nodes passing through the nucleus
What is the shape of f orbitals?
3 nodes that pass through the nucleus
What is the order of electron configuration?
1s2 2s2 2p6 3s2 3p6 4s2 3d8 4p6
What are ions with unpaired electrons?
Paramagnetic
What are ions without unpaired electrons?
Diamagnetic