Atomic Structure and Calculations
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
what is an isotope (1)
atoms with the same number of protons but different number of neutrons
What did Jason dalton discover (2)
Atoms are spheres
Each element is made up from different spheres
What did Jj Thompson discover (3)
The electron
That atom was made up of other particles
Developed plum pudding model
What did Ernest Rutherford discover (4)
The nucleus, it was very small and positively charged
Concluded atoms were made up of mostly empty space
Fired Alpha particles at gold leaf, which went through, proving it was mostly empty space
Some deflected proving the positive charge in centre
What did Bohr discover (1)
Electrons traveled in orbitals
what are the 4 steps of time of flight spectrometry (4)
ionisation
acceleration
flight tube
detection
what are the two ways to ionise an atom (2)
electron impact
electrospray ionisation
explain what electron impact is (4)
a vaporised sample is injected at low pressure
an electron gun fires high energy electrons at the sample
this knocks out an outer electron
this forms positive ions with different charges
what type of elements is electron impact used for (1)
elements/ substances with a low formula mass
what is a limitation of electron impact (1)
can cause larger ions to fragment
explain electro spray ionisation (5)
the sample is dissolves in a volatile, polar solvent
its injected through a fine needle giving a fine mist or aerosol
the tip of needle has high voltage
the sample gains a proton at the tip of needle
the solvent evaporates away while the ions move through towards a negative plate
why is electro spray ionisation used for larger organic molecules (2)
is a softer technique
so it does not cause fragmentation
what is a mass spectrometer used for (2)
to determine all the isotopes present in a sample of an element
to therefore identify the element
why does a spectrometer need to be in a vacuum (1)
otherwise air particles would ionise and register on the detector
explain the relationship between mass and velocity (1)
lower mass means faster velocity
KE equation;
KE = ½ mv2
time, distance and velocity
t = d/v
velocity in terms of KE;
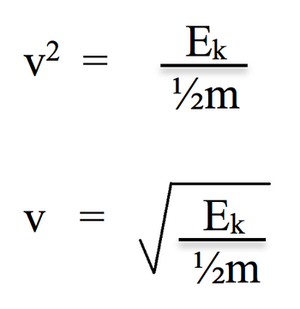
what occurs in the flight tube in spectrometry (2)
the positive ions with smaller mass will move faster than the larger mass ones as they all have the same kinetic energy
the ions are distinguished by different flight times
how are the ions detected in spectrometry (1)
ions reach negative plate which generates a small current which is fed to a computer for analysis
how is relative abundance determined in mass spectrometry (1)
the size of the current is proportional to the abundance of the species
relative atomic mass equation ;
RAM = total (isotopic mass X % abundance) / 100
name the sub energy levels and how many electrons they hold (4)
s- 2 electrons
p- 6 electrons
d- 10 electrons
f- 14 electrons
what is the electronic structure of the d-block elements (1)
when d-block elements form ions, they lose electrons from 4s electrons first
what is the definition of first ionisation energy (1)
is the enthalpy change when one mole of a gaseous atom forms one mole of gaseous ions with a single positive charge
what is the definition of second ionisation energy (2)
the enthalpy change when one mole of gaseous ions with a single positive charge forms when one mole of gaseous ions with a double positive charge
what are the factors that affect first ionisation energy (3)
the attraction of the nucleus
the distance of the electrons from the nucleus
shielding of the attraction of the nucleus
how does attraction from the nucleus affect the ionisation energy (1)
the greater the attraction between the nucleus and outer electron, the higher the ionisation energy will be
how does the distance of the electron from the affect the ionisation energy (1)
the bigger the atom the further the outer electrons are from the nucleus and the weaker the attraction to the nucleus
how does shielding affect the ionisation energy (1)
the electron in an outer shell is repelled by electrons in complete inner shells, weakening the attraction of the nucleus
why are successive ionisation energies always larger (3)
when the first electron is removed, a positive ion is formed
the ion increases the attraction on the remaining electrons
so the energy required to remove the next electron is larger
what is periodicity (1)
a repeating pattern across a period
why do first ionisation energies decrease down a group (2)
the outer electrons are found in shells further from the nucleus
and are more shielded so the attraction of the nucleus becomes smaller
why is there a general increase in the first ionisation energy across a period (2)
the electrons are being added to the same shell which has the same distance from the nucleus and same shielding effect
the number of protons increase, making the attraction of the nucleus greater
define avogadro’s number (1)
one mole of any specified entity contains 6.022 X 1023 of that entity
define relative atomic mass (1)
average mass of one atom compared to one twelfth of the mass of a carbon 12 atom
define relative molecular mass (1)
the average mass of a molecule compared to one twelfth of the mass of a carbon 12 atom
define mole (1)
the amount of substance in grams that has the same number of particles as there are atoms in 12 grams of carbon 12
moles equations;
concentration = moles / volume
moles = mass / mr
how do convert degrees into kelvins (1)
add 273
gas equation ;
PV = nRT
where R is 8.31
density;
density = mass / volume
define the empirical formula (1)
the simplest ratio of atoms of each element in a compound
define molecular formula (1)
the actual number of atoms of each element in the compound
percentage yield ;
percentage yield = (actual yield / theoretical yield) X 100
percentage atom economy ;
percentage atom economy = (mass of useful products / mass of all reactants) X 100
why do chemists want a high percentage yield (1)
it means there has been efficient conversion of reactants to products
why do chemists want high atom economy(1)
so that the maximum mass of reactants ends up in the desired product
state the features of the current model that are not shown in the rutherford model (2)
current model includes neutrons and protons whereas rutherford model does mot include either
current model shows electrons in different orbitals whereas the rutherford model does not
define the mass number of an atom (1)
number of protons and neutrons
give two reasons why it is necessary to ionise the isotopes of an element before they can be analysed in a TOF mass spectrometer
ions will interact with and be accelerated
ions create a current when hitting the detector
describe how molecules are ionised using electrospray ionisation
sample is dissolved in a volatile solvent
injected through needle at high voltage
each molecule gains a proton
give the first ionisation energy equation for Fe
Fe(g) —> Fe+(g) + e-
the first ionisation energies of the elements in Period 2 change as the atomic number increases.
explain the pattern in the first ionisation energies of the elements from lithium to neon (6)
1st IE increases
due to proton increase and electrons are in same shell
therefore the elements have similar shielding therefore stronger attraction between nucleus and outer e-
B lower than Be
as its outer electron is in 2p
O is lower than N
as 2 electrons in 2p need to pair and pairing causes repulsion
give 2 differences between the plum pudding model and the modern model of atomic structure (2)
nucleus in modern model contains protons and neutrons
electrons in modern model are arranged in orbitals
explain which one of the elements Mg/Al has lower first ionisation energy (3)
Al
outer electron in 3p sublevel
higher in energy as its further from nucleus so easier to remove
state and explain if the chemical properties of isotopes differ (2)
no difference in chemical properties
because all isotopes have the same electron configuration
state and explain the trend in the 1st IE of the elements in group 2 from magnesium to barium (3)
IE decreases
as atoms get bigger due to more energy shells
therefore weaker attraction of ion to lost electron
explain why it is necessary to ionise molecules when measuring their mass in a TOF mass spectrometer (2)
only ions will interact with and be accelerated by an electric field
only ions will create a current when hitting the detector
outline how the TOF mass spectrometer is able to separate two species to give two peaks (4)
positive ions are accelerated by an electric field
to a constant KE
the positive ions with lower m/z move faster than those wit greater m/z
therefore they arrive first at the detector
state and explain the effect that rinsing a burette will have on the value of the titre (2)
titre would increase
as solution will be more dilute
explain the effect on the Mr caused by liquid in a gas syringe not vaporising (2)
calculated mr will be greater than actual
as mass recorded higher than mass of gas
what is the importance of percentage yield
allows you to calculate the max amount of product possible in the reactions
what is the importance of atom economy
allows you to minimise the amount of by products by maximising the mass of atoms that end up in the desired product
define the term concordant titres
titres that are within 0.1cm3 of each other
suggest the reasons, within an gaseous experiment, why the Mr of value determined from the experimental results differ from the actual Mr
apparatus inaccuracy
volume of gas syringes is greater than true volume therefore if V is too large, Mr is too small
the temp measures is less than the temp of the gas in the syringe therefore if T is too small, Mr is too small
measured mass of liquid transferred to the syringe is less than the actual mass transferred
suggest and explain a safety precaution that the student should take when using a toxic gas in an experiment (2)
carry out in a fume cupboard
to avoid toxic vapour