Chemistry Unit 1, Atomic composition and isotopes
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Where is a proton located; what is its charge relative mass
In the nucleus; charge 1; mass 1
Where is a neutron located; what is its charge and relative mass
In the nucleus; charge 0; mass 1
Where is an electron located, and what is its charge and relative mass?
In the electron cloud; charge -1; mass ≈0
What is the atomic number of an element?
Number of protons; determines the element
What is the mass number of an atom?
Total number of protons + neutrons
How do you calculate the charge of an ion?
Charge = protons – electrons
Define an ion.
An atom that has gained or lost electrons, giving it a net charge; atom/s with electical charge
Define an isotope.
Atoms of the same element with different numbers of neutrons
Write isotopic notation for an atom with 6 protons and 8 neutrons.
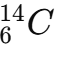
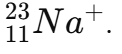
Calculate protons, neutrons, electrons for
P = 11, N = 12, E = 10
How do you find the number of neutrons in an atom?
Neutrons = Mass number – Atomic number
How do you know the number of electrons in a neutral atom?
Equal to the number of protons
What is an isotope
Atoms of the same element with the same number of protons but different numbers of neutrons.