IB Chem Option B: Biochemistry (SL)
1/127
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
128 Terms
Metabolism
the sum of chemical reactions occurring in a living organism
Metabolic pathways
reactions are controlled in sequences and cycles, the product of each stem is the reactant in the next
Metabolites
compounds taking part in metabolism
Enzymes
biological catalysts
Coupled metabolic reactions
the energy from one reaction is used to drive another
Anabolism
building/synthesis of molecules from smaller organic molecules
products are larger/more complex/of a higher energy
generally require an input of energy (ie. endothermic)
Precursors
reactants of anabolism
Catabolism
metabolic reactions of breakdown/degradation
releases energy, produces energy-poor end products
usually energy (ATP) produced from catabolic reactions is used to drive anabolic reactions — energy coupling
Futile cycles
when stable complex structures cannot be formed because they are broken down as they are formed
occurs when catabolic and anabolic reactions are not controlled by separate metabolic pathways
Trace elements required by living things
S, P, Ca, Fe — required in small amounts
Macromolecules
large complex biomolecules composed of monomers
relative molecular masses of several thousand
Condensation polymers
polymers whose synthesis involves a loss of H2O per covalent bond
Polymerases
enzymes which catalyse condensation reactions
Hydrolysis reactions
H2O molecule added for each covalent bond removed
catalysed by enzymes, may be favoured by heat or acidic/alkaline conditions
Examples of structure=function in biomolecules
collagen and cellulose — tough and insoluble
enzymes — shape of active site
nucleotide —store/transmit genetic information by sequences of nitrogenous bases
Outline photosynthesis
acts as a “carbon sink”
captures solar energy using chlorophyll and uses it to synthesize energy-rich biomolecules
Light energy drives a series of redox reactions in which water is split into hydrogen and oxygen
hydrogen ultimately reduces CO2 to simple sugars
essentially, photosynthesis transforms energy poor reactants to energy-rich glucose with the release of O2
Outline cellular respiration
acts as a carbon source
glycolysis releases a small proportion of glucose’s energy (anaerobic respiration stops here)
in aerobic conditions, it involves cytochromes, which are successively reduced then re-oxidized
oxygen is a terminal electron acceptor — when it is reduced to H2O
Fibrous proteins
structural components
elongated molecules with dominant secondary structure
water insoluble
Globular proteins
tools that operate at the molecular level — as enzymes, carriers, receptors
compact spherical molecules with
dominant tertiary structure
water soluble
2-amino acid condensed structural formula
NH3-CHR-COOH
Amine-variable R group-carboxyl group
R-groups on amino acids
variable - around 20
Amino acids can be classified according to the chemical nature of their R group,
usually based on their different polarities
Amino acids physical properties
crystalline compounds with high melting points, usually above 200 °C, and they have much greater solubility in water than in non-polar solvents
move in an electric field
These properties are all typical of ionic compounds; suggesting that amino acids contain charged groups.
The charges are a result of acid-base behaviour
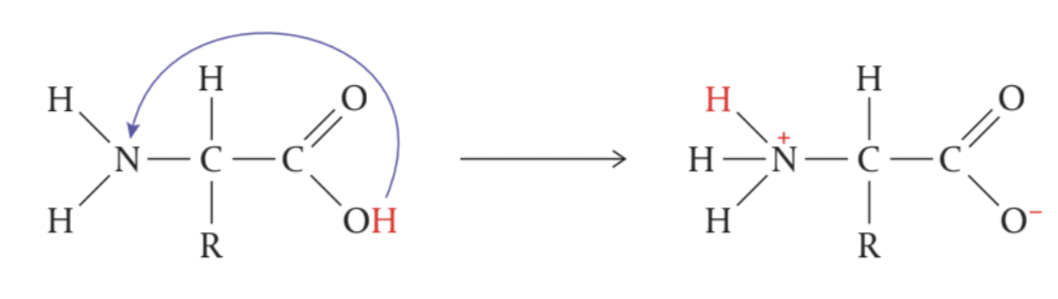
In aqueous solution and in crystalline form, amino acids commonly exist with both positive and negative charges within the molecule, known as zwitterions.
They are sometimes referred to as internal salts, as the charges result from an internal acid-base reaction
with the transfer of a proton (H+) from the acid – COOH group to the basic –NH2 group in the same amino acid.
Amino acids acid/base properties
contain both an acidic group and a basic group, they are amphoteric or amphiprotic
In aqueous solution they will accept and donate H+ according to changes in the pH of the medium
in the zwitterion it is the conjugates of the acid and the base that are
responsible for this property.
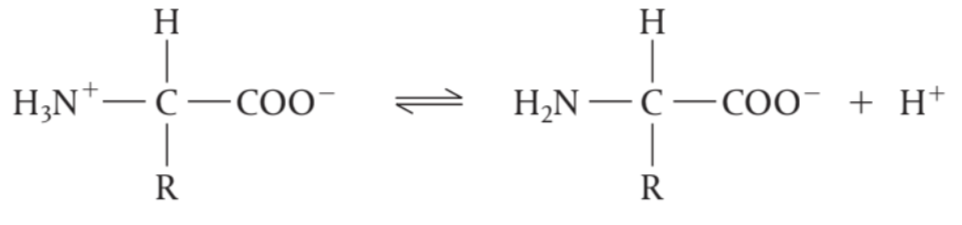
Amino acids can act as a Brønsted-Lowry acid
at high pH (low [H+]), this reaction is favoured as the –NH3+ group loses its H+ and
forms an anion
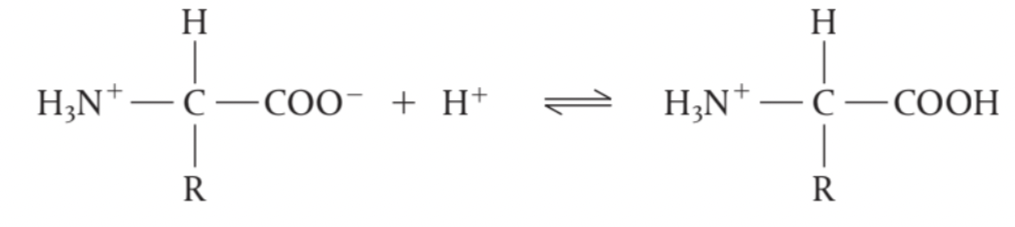
Amino acids can act as a Brønsted-Lowry base
at low pH (high [H+]), this reaction is favoured as the –COO– group gains H+ and
forms a cation.
Isoelectronic point of amino acids
The charge carried by an amino acid depends on the pH of the medium
The isoelectric point is the intermediate pH at which it is electrically neutral.
With no net charge at this pH, amino acids will not move in an electric field.
the molecules will have minimum mutual repulsion and so be the least soluble at this pH.
Amide link/Peptide bond
a molecule of water is eliminated and a new bond is formed between the acid group of one amino acid and the amino group of the other.
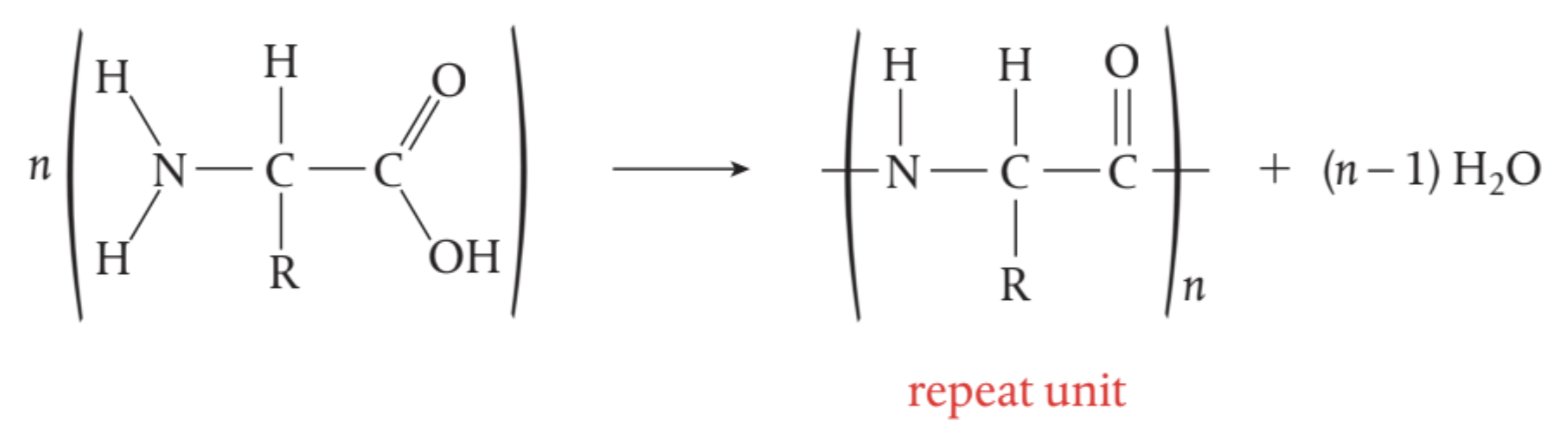
general equation for the synthesis of a polypeptide from its amino acids
structure and therefore the function of a protein is determined by…
the sequence of amino acids in the polypeptide — the placements of amino acids /w diff R groups in the chain affects its structure and reactivity
Protein primary structure
number and sequence of amino acids in its polypeptide chain held together by peptide bonds
forms the covalent backbone of the molecule— once the primary structure has been determined, all the other levels of protein structure follow
Secondary protein structure
refers to the folding of the polypeptide chain as a result of hydrogen bonding between peptide bonds along its length
Hydrogen bonds can form between the
–C=O group of one peptide bond and the
–N–H group of another
peptide bond further along the chain which will cause the chain to fold.
α-helix
regular coiled configuration of the polypeptide chain—hydrogen bonds forming between two peptide bonds four amino acid units apart
This twists the chain into a tightly coiled helix— 3.6 amino acids per turn
flexible and elastic as the intra-chain hydrogen bonds easily break and re-form as the molecule is stretched
β-pleated sheet
structure composed of ‘side by side’ polypeptides which are in extended form
not tightly coiled as in the α-helix
arranged in pleated sheets that are cross-linked by inter-chain hydrogen bonds
is flexible but inelastic
tertiary structure
further twisting, folding, and coiling of the polypeptide chain as a result of interactions between the R groups, known as side chains.
structure that results is a very specific compact three-dimensional structure, known as the protein’s conformation.
most stable arrangement of the protein
the interactions between the side chains are all intra-molecular forces, as they occur within the one polypeptide chain.
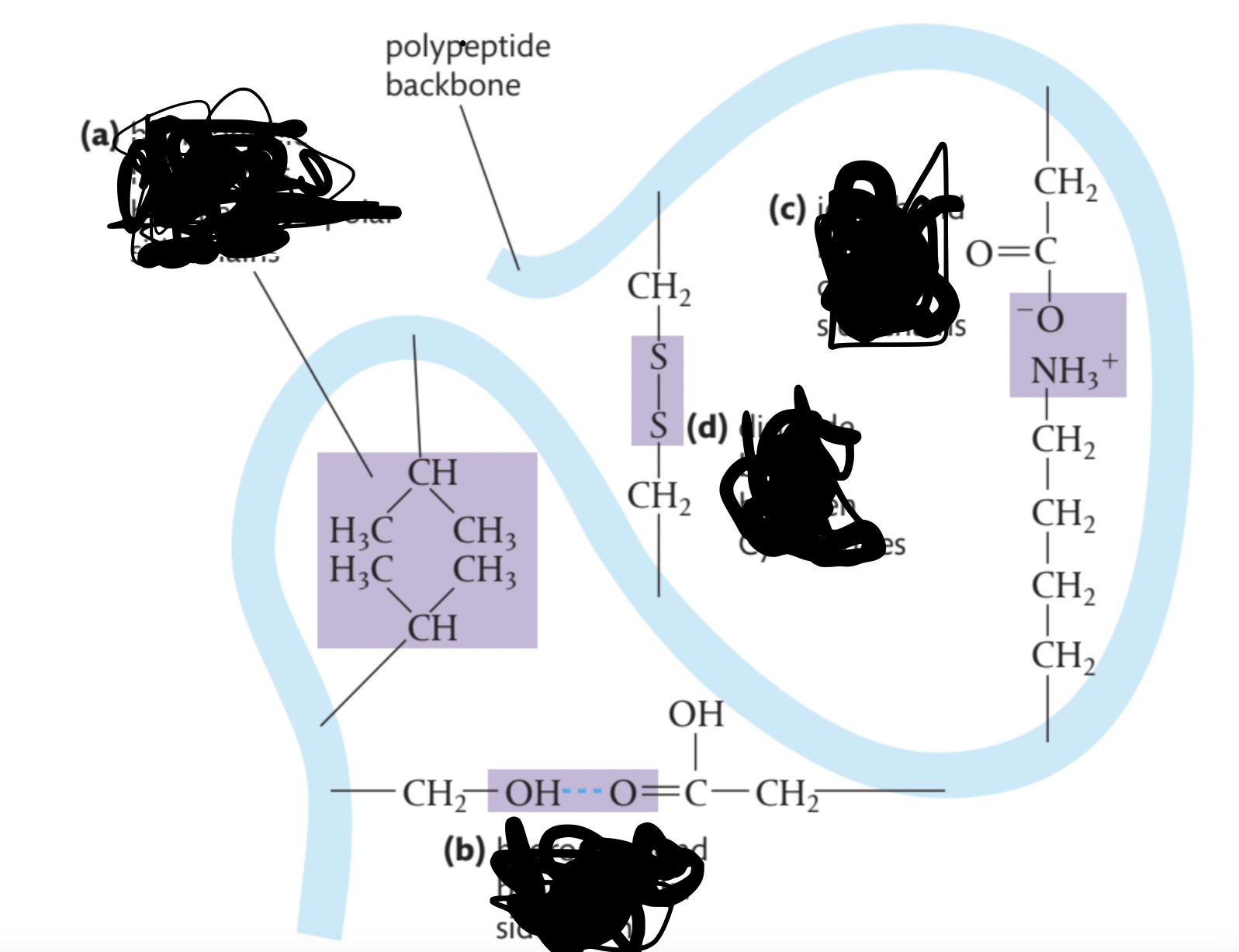
Indicate which bonds form at each letter
a) hydrophobic interactions between non-polar side chains
b) hydrogen bond between polar side chains
c) ionic bond between charged side chains
d) disulfide bridge between Cys residues
Denaturing
When a protein loses its specific tertiary structure as a result of disruptions
eg. heat, presence of metal ions, pH changes
Quaternary structure
proteins that consist of more than one polypeptide chain
This association involves similar forces and bonds to those found in the tertiary structure – hydrophobic interactions, hydrogen bonds, ionic bonds, and disuldide bridges.
Collagen quaternary structure
a triple helix of three polypeptide chains, with inter-chain hydrogen bonds between them.
This helps to give it a stable rope-like structure that is resistant to stretching.
Haemoglobin structure
made up of four polypeptide
chains that fit together tightly in the protein
assembly
each carry an iron-containing haeme group
relative energy of lipids compared to carbs
lipids contain more energy per gram given they are more reduced molecules
their ration of H to O is greater
as a result, they yield more energy when oxidized
a gram of lipid releases almost twice as much energy as a gram of carbohydrate
Lipids as energy storage
although lipids have more energy per gram than carbohydrates, lipids are non-polar (water insoluble) whereas carbohydrates are polar (water soluble)
so more reactions are involved in their breakdown —their energy is released more slowly
The fat stores in animals, known as adipose tissue or blubber, serve as reservoirs of energy, swelling and shrinking as fat is deposited and withdrawn.
Plants also sometimes store lipids for energy, for example as oils in seeds.
Purposes of stored fat
energy storage
Stored fat helps to protect some body organs, such as the kidneys, and a layer of fat
under the skin acts as a thermal insulator.
Lipids also act as electrical
insulators —myelin sheath in nerve cells gives electrical insulation and speeds up nervous transmission
Atherosclerosis
restricted blood flow due to the deposition of lipids on arterial walls given their low solubility
associated with high blood pressure and can lead to heart disease
Obesity causes and consequences
because of the body’s ability to convert excess fats into adipose tissue, a diet too rich in lipids can lead to the excess accumulation of body fat
is linked to diabetes and a variety of cancers
Describe the transport of cholesterol in the blood
transported bound in different lipoproteins: high-density lipoproteins (HDLs); low-density lipoproteins (LDLs)
high levels of LDL cholesterol are associated with increased deposition in the walls of
the arteries
high levels of HDL cholesterol seem to protect against heart attack —tends to carry cholesterol away from the arteries, so slowing
its build-up.
Sources of LDL cholesterol
saturated fats and trans fats
polyunsaturated fats sources and role in the diet
fish, nuts, corn oil, etc
considered beneficial in lowering levels of LDL cholesterol
essential fatty acids
those which cannot be manufactured by the body so must be digested
eg. omega-3-poly-unsaturated fatty acid
omega-3-poly-unsaturated fatty acid sources and benefits
found in fish oils and flax seeds
be linked with reduced risk of cardiovascular disease as well as with optimum neurological development.
lipids in the form of ________ are found in hormones
steroids
Female steroid hormone examples
Used in contraceptive pill formulations
Used in HRT (hormone replacement therapy) sometimes prescribed during menopause
Male steroid hormones name and uses
androgens — testosterone is the most important
aka anabolic steroids as they are involved in promoting muscle tissue growth
Synthetic forms of them are used medically to help gain weight after debilitating diseases
used as performance-enhancing drugs by athletes —can increase strength and endurance.
Structure of triglycerides
composed of a glycerol molecule covalently bonded to three fatty acids — called an ester linkage
Involves a condensation reaction called an esterification reaction, yields three H2O when the COOH and OH group bond
In most natural oils and fats the three fatty acids that form one triglyceride molecule
are not all the same. They can be designated R1, R2, and R3
differ by length of tail and degree of saturation
Glycerol structure (IUPAC name)
three carbons, each with a hydroxyl group: propane-1,2,3-triol
Saturated fatty acids structure (VSEPR, IMFs) and examples
CnH2n+1COOH
The carbon chain is made
from C-C single bonds
tetrahedral bond angles (109.5°) so molecules to pack relatively closely together.
so strong LDFs and relatively high BPs, their triglycerides are solid at SATPT
Known as fats - eg butter/lard
Unsaturated fatty acids structure and examples
containing one or more C-C double bonds and 120° bond angles,
have kinks in the chains—molecules can’t pack closely
form unsaturated triglycerides which have weaker intermolecular forces and lower melting points
liquid at SATP
They are known as oils and are found mostly in plants and fish.
eg. corn oil and cod liver oil.
Trends in BP in fatty acids
Generally, the melting points increase with increasing molar mass (length of the hydrocarbon chains) and with increasing degree of saturation.
Iodine number definition and explanation
Unsaturated fatty acids undergo addition reactions by breaking the C=C bond and adding incoming groups to the Cs
This occurs with I2
one mole of iodine will react with each mole of double bonds
in the fat
iodine number, defined as the number of grams of iodine which reacts with 100 grams of fat — measures degree of unsaturation
Hydrogenation of fatty acids
increases saturation of fatty acids
partial hydrogenation yields trans fats — C’s are on either side of the double bond
Hydrolytic rancidity
fat breaks down by hydrolysis reactions, using the water in food.
The site of reactivity is the ester linkages in the triglycerides.
Conditions: occurs more readily in heat (eg. deep-fat frying) — catalyzed by lipase —favoured in the presence of certain bacteria
hence can be reduced by refrigeration
the rancid smell and flavour is due to the release of free fatty acids, such as butanoic and octanoic acids which are released from rancid milk
Oxidative rancidity
unsaturated fats react with oxygen from the air — auto-oxidation
The site of reactivity is the C=C bond
The products responsible for the rancidity are volatile aldehydes and ketones.
accelerated by light and enzymes or metal ions
proceeds via a free-radical mechanism and so yields a mixture of products.
characteristic of fats and oils that have a high proportion of C=C bonds
Can be controlled by addition of antioxidants
Relative stability of saturated and unsaturated fats
As they cannot undergo auto-oxidation, saturated fats are more stable than unsaturated fats
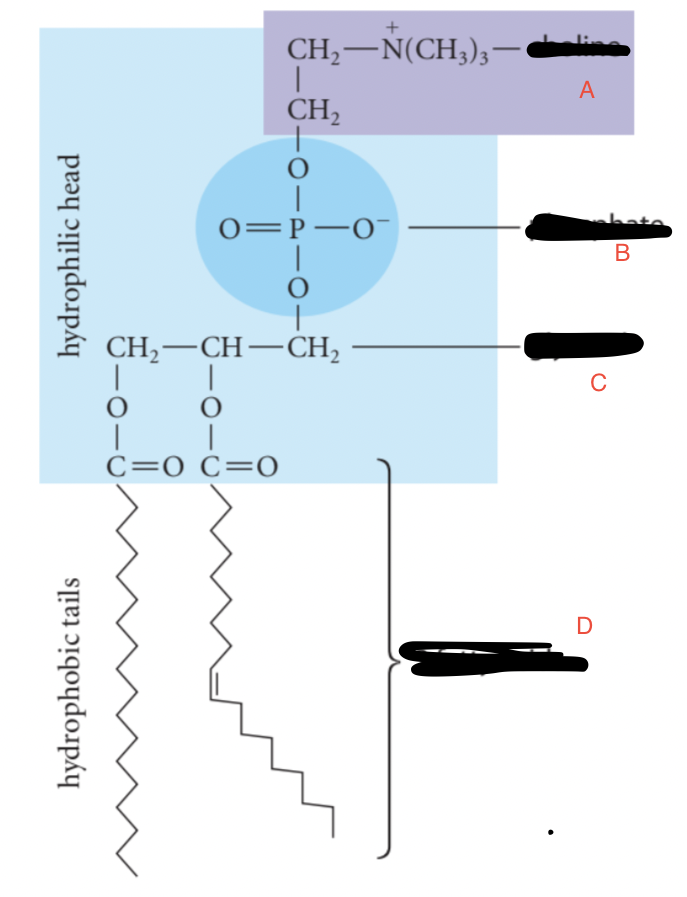
Label the phospholipid —lecithin
A - Choline
B - Phosphate
C - Glycerol
D - 2 Fatty Acids
Phospholipid varieties differ by
the group attached to the phosphate
fatty acid chain
Phospholipid bilayer
Spontaneously form a bilayer due to the phosphate head being hydrophilic and the fatty acid tails being hydrophobic
maximizes the interactions between the polar groups and water, while creating a non-polar, hydrophobic interior.
Saponification reactions
reverse of esterification reactions
used in soap production
Alkaline hydrolysis produces the salt of the fatty acid
Conditions for hydrolysis of triglycerides and phospholipids
can occur in acidic or alkaline conditions, or catalyzed by enzymes known as lipases
This occurs during the digestion of lipids in the gut, where the activity of the enzymes is controlled largely by local changes in pH.
Steroids structure
lipids with a structure consisting of four fused rings, known as a steroidal backbone
Cholesterol functions
used as a precursor in the synthesis of many biomolecules including other steroids such as sex hormones, bile acids, and Vitamin D.
component of cell membranes as it helps to provide fluidity and permeability to the structure
The hydroxyl group interacts with the polar head groups of phospholipids in the membrane, while the non-polar rings and hydrocarbon chain interact with the hydrophobic tails of the phospholipid bilayer
Carbohydrates general formula + explanation
Cx(H2O)y
oxygen and hydrogen are always in the same ratio as water
carbohydrate = hydrated carbon
Carbohydrate monomers
monosaccharides
Carbohydrate polymers
polysaccharides
Monosaccharide functions
soluble in water — taken up by cells rapidly
used as the main substrate for respiration, releasing energy for all cell processes.
act as precursors in a large number of metabolic reactions, leading to the synthesis of other molecules such as fats, nucleic acids, and amino acids.
Polysaccharide functions
are insoluble, used as the storage form for carbohydrates
Animals use glycogen as storage in the liver and muscles
plants store starch in cells
energy reserves can be broken down into
monosaccharides, which are then oxidized in respiration to release energy for the cell’s
activities
cellulose is used structurally in plants
Monosaccharides empirical formula
CH2O
Monosaccharides functional groups
2+ hydroxyl groups (water soluble)
carbonyl group (aldehyde or ketone)
Monosaccharides ring vs straight chain structures
In aqueous solution these sugars undergo an internal reaction resulting in the more familiar ring structures
Haworth projection formulas
representations of the ring forms of sugars
The edge of the ring nearest the reader is represented by bold lines, and the letter C for the carbons in the ring are usually omitted from the structure.
glycosidic linkage
bonds between monosaccharides
involves a condensation reaction: two OH- groups on different sugars bond to form one molecule of H2O
Disaccharides properties
soluble molecule
can be hydrolysed into two monosaccharides by acid hydrolysis or by enzyme-catalysed reaction.
Combining different monosaccharides will produce different disaccharides.
polysaccharides differ by…
isomer of glucose used and amount of cross-linking in the chain
Micronutrients
needed in extremely small amounts, generally less than 0.005% of body mass
are usually measured in mg or μg per day.
These substances are needed to enable the body to produce enzymes, hormones, and other biomolecules.
Absence of micronutrients can →
deficiency diseases
Vitamins
organic compounds, needed in small amounts for normal growth and metabolism, which (with the exception of vitamin D) are not synthesized in the body.
They are usually broken down by the reactions in which they are involved, so must be taken in from suitable food sources in the diet. They are often classifed according to their relative solubility in water or in lipid.
Classification of vitamins
They are often classified according to their relative solubility in water or in lipids.
Water-soluble vitamins
have polar bonds and the ability to form hydrogen bonds with water.
They are transported directly in the blood
and excesses are filtered out by the kidneys and excreted.
Vitamins B and C are water soluble.
Lipid-soluble vitamins
mostly non-polar molecules with long hydrocarbon chains or rings.
They are slower to be absorbed
excesses tend to be stored in fat tissues where they can produce serious side-effects.
Vitamins A, D, E, and K are fat soluble.
Vitamin A (Retinol) solubility and properties
fat soluble
The hydrocarbon chain and ring are non-polar and influence the solubility more than the one – OH group
involved in the visual cycle in the eye, and particularly important for vision in low light intensity
Vitamin C (Ascorbic Acid) solubility and properties
water soluble
several – OH groups enable hydrogen bonds to form with water
acts as cofactor in some enzymic reactions,
important in tissue regeneration following injury
and resistance to some diseases
contains several functional groups (– OH and – C=C –) that are relatively easily oxidized —vitamin is easily destroyed by most methods of food processing and storage —best obtained from fresh fruits and vegetables.
Vitamin D (calciferol) Solubility and Properties
fat soluble
predominantly a hydrocarbon molecule with four non-polar rings and only one – OH group
chemically similar to cholesterol
stimulates the uptake of calcium and phosphorous ions by cells from small intestine and important in the health of bones and teeth (those ions are involved in bone mineralization)
Sensitivity of vitamins to heat
water-soluble vitamins, eg C, are most sensitive to heat
but other vitamins also lose some activity after being heated.
causes of malnutrition arising from vitamin deficiency
• lack of distribution of global resources
• depletion of nutrients in the soil and water
• lack of education about, or understanding of, the importance of a balanced diet
• over-processing of food for transport and storage
• the use of chemical treatments such as herbicides in food production.
possible solutions to the varying challenges of malnutrition
• the fortification of different staple foods with micronutrients
• the availability of vitamin supplements in many forms
• the possible improvements to nutrient content of food through genetic modification
• increased labelling of foods with content information
• education regarding the nature of a balanced diet and promotion of the importance of personal responsibility in dietary choices.
Xenobiotics
chemical compounds present in an organism that are foreign to them
Also describes chemicals found in organisms in higher-than-normal concentrations and compounds that are not produced naturally
but only by synthetic processes
eg. drugs, some hormones, insecticides, heavy metals, plastics, food additives, pollutants
Describe the successful metabolization of non-polar xenobiotics
non-polar xenobiotics diffuse passively into the cell
may be modified by enzymes and then detoxified in the cell
This describes how drugs are metabolized as well as pesticides in plants
may lead to resistance to the effect of the chemical in some cases
Bioaccumulation
occurs when a xenobiotic cannot be modified in the organism so it builds up in the cell —its concentration increases in an organism
eg. mercury poisoning is caused by the build-up of methylmercury in the brain
pharmaceutically active compounds effects in the environment
eg. antibiotics, painkillers, and chemotherapy drugs
may be discharged from industries
or hospitals, or passed through the human body and released unmodified or partially metabolized in urine
Sewage treatment plants may break the xenobiotic down through bacterial action, but often this process is incomplete
the compounds are released and can be taken in by fish
There is some concern about male fish becoming feminized (ie. unable to reproduce) due to female estrogen present in sewage water from individuals who take the synthetic contraceptive pill
Biomagnification
the increase in concentration of a xenobiotic in a food web
due to the lack of enzymes to break them down — harmful substances produced by biological processes are broken down and hence don’t build in concentration in the environment
if a xenobiotic cannot be metabolized, it is passed along a food chain through feeding, affecting animals at the top of the food chain to the greatest extent
Describe the biomagnification of DDT
It was used as an insecticide to control mosquito-spread diseases
it is not able to be broken down by organisms and is fat soluble so it bioaccumulates in tissue and is passed along a food chain
because its concentrations increased along trophic levels, birds of prey eg. ospreys experienced the greatest consequences: the thinning of their eggs led to a decrease in population