UPENN NEUR2600: Wk08 Videos
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
List examples of innate immunity.
peristalsis
mucosal secretions
coughing
gastric acid
Modulated by stress hormones and ANS.
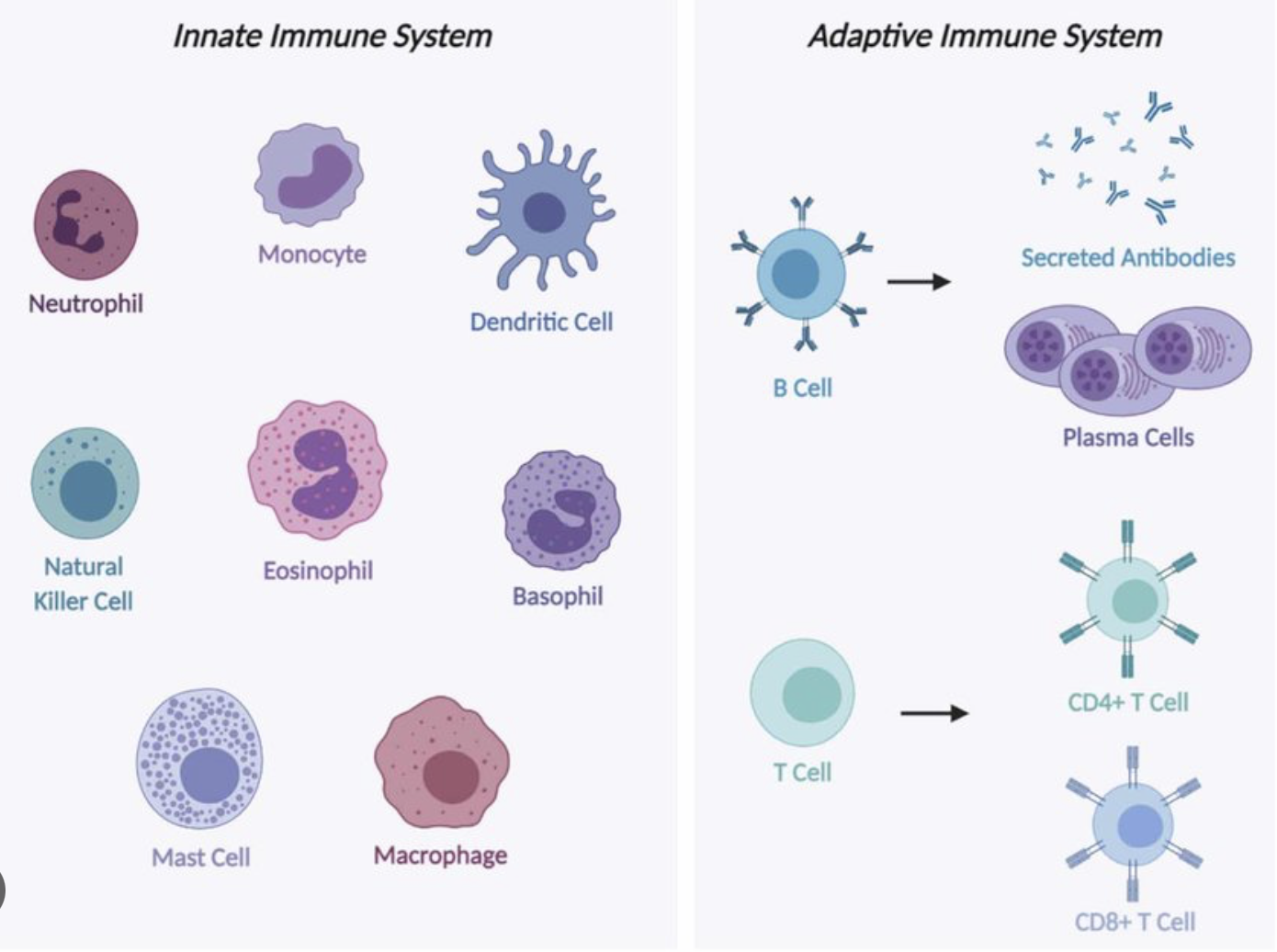
List examples of specific immunity.
T cells and B cells. Recognition of specific foreign substances or pathogens.
Where are immune cells generated?
All of them are produced in the bone marrow. T cells mature in the thymus.
List immune cells and their primary function(s).
B cells .. humoral adaptive immunity
T cells .. cell-mediated adaptive immunity
Neutrophils .. eat bacteria, fungi; first responders
Dendritic cells .. present antigens (Ag) to T cells
Macrophages .. eat pathogens, debri; present Ag
Eosinophils .. target multicellular parasites
Basophils .. assist in killing parasites (histamine, etc)
NK cells .. destroy virally infected cells; tumour cells
Mast cells .. histamine; against parasites; again mucosal pathogens
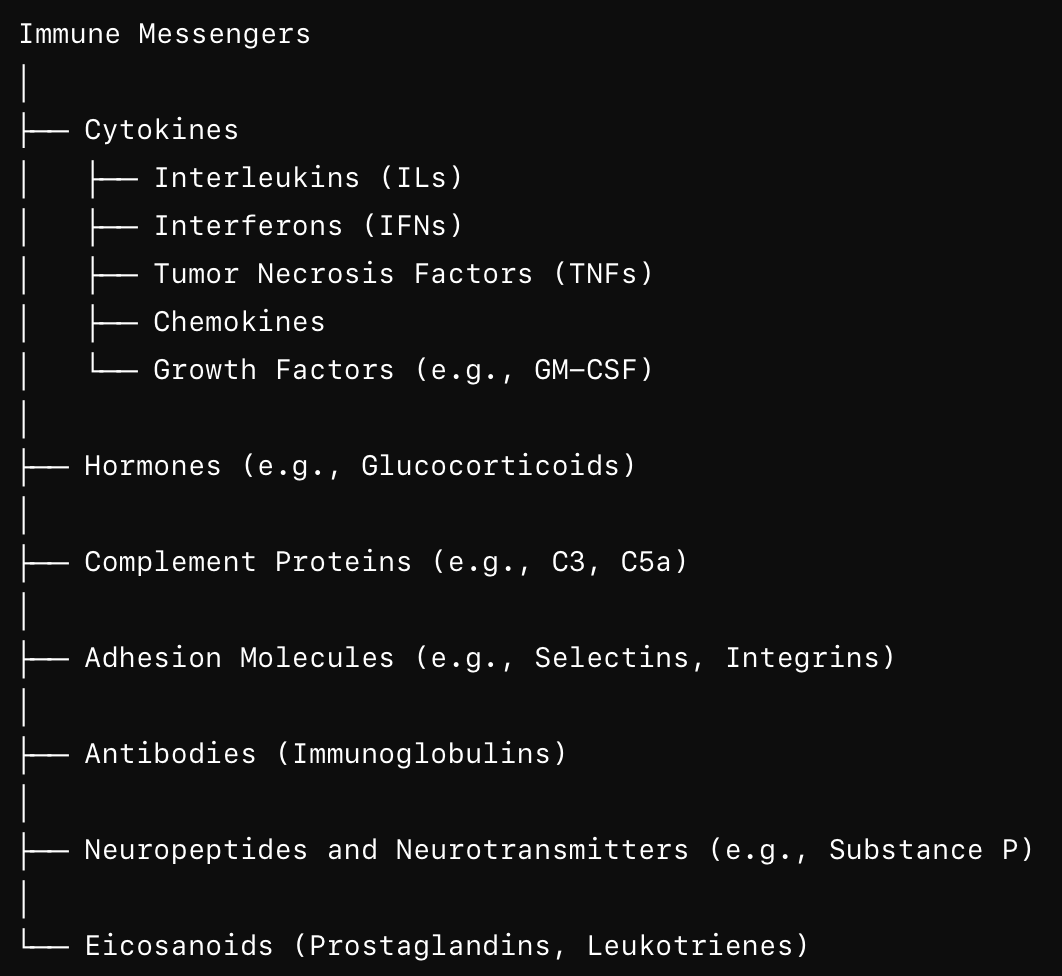
List classes of immunity related messengers and their roles.
Cytokines are small molecules release by (mostly) immune cells. Mediate inflammation, immunity. Include ILs, IFNs, TNFs, TGFs, CSFs (colony-stimulating factors).
Other molecules:
Chemokines .. induce chemotaxis in nearby cells.
Growth factors (GFs) .. cell growth, differentiation
Adhesion molecules
Hormones
Antibodies
Complement proteins
Neuropeptides, neurotransmitters
Eicosanoids (prostaglandins, leukotrienes)
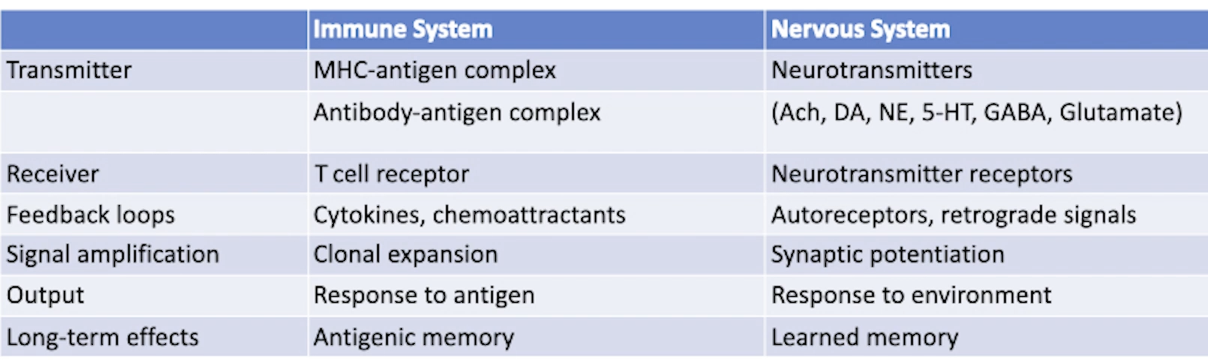
Compare immune and neuroendocrine system in terms of their transmitter, receiver, feedback loop, signal amplification, output, long-term effect.
Are we more likely to get sick if we’re stressed? Why?
Yes.
Cortisol suppresses the immune system (evolutionary - to prioritise glucose for brain/muscles for survival).
Explain how our ANS innervates lymphocytes and why?
lymphocytes are T cells, B cells, NK cells
lymphocytes are not directly innervated by neurons
by ANS can release NT in proximity e.g. in the spleen, bone marrow, lymph nodes
NE binds to beta-adrenergic receptors on lymphocytes
ACh binds to nicotinic and muscarinic receptors
SNS influences cytokine production, affects movement and distribution, affects growth and specialisation of lymphocytes
PNS suppresses pro-inflammatory cytokines, modulates other functions
Cortisol (HPA) suppresses immune cells
Why
scales immune system during stress
conserves energy during rest & digest period
ANS can prevent overactivation of the immune system
SNS can increase activation e.g. during infection
In general how does SNS / fight or flight affect the immune system?
immediate stress response increases the activity of the immune system; e.g. NE (e.g. in the spleen, bone marrow, lymph nodes) can stimulate mobility, growth, specialisation and pro-inflammatory cytokines in lymphocytes
prolonged stress response activates HPA and increases cortisol which suppresses the immune system; which may be require to conserve energy and to prevent overactivation of the immune system / autoimmunity
Note that PNS activation opposes this mobilisation by reducing pro-inflammatory cytokines, etc.
How would beta blockers affect the immune function?
beta blockers will block beta-adrenergic receptors on lymphocytes
this will reduce pro-inflammatory cytokines
this will reduce lymphocyte activation and trafficking
it may reduce chronic inflammation
however this may also impair body’s abilities to fight infections
What neurons does PVN (HT) have? What receptors? Functions of PVN?
PVN neurons
magnocellular part (ADH and oxytocin)
parvocellular part (CRH and TRH)
DA neurons (modulate PRL release, other functions)
autonomic neurons (to other brain areas, to spine)
Receptors & inputs
leptin, insuli, GLP-1, OXM => energy, satiety
CCK (via NTS) => energy, satiety
ArgRP, POMC (via ARC) => energy, satiety
Electrolytes, angiotensin, relaxin => control ADH
Hippocampal inhibition => CRH response
SCN => levels of lighting => CRH / pineal / food intake
Functions
Stress response (HPA; spine, brainstem, spleen, etc)
Autonomic functions (HR, BP, digestion)
Feeding behaviour
Syncing feeding / HPA with circadian rhythms
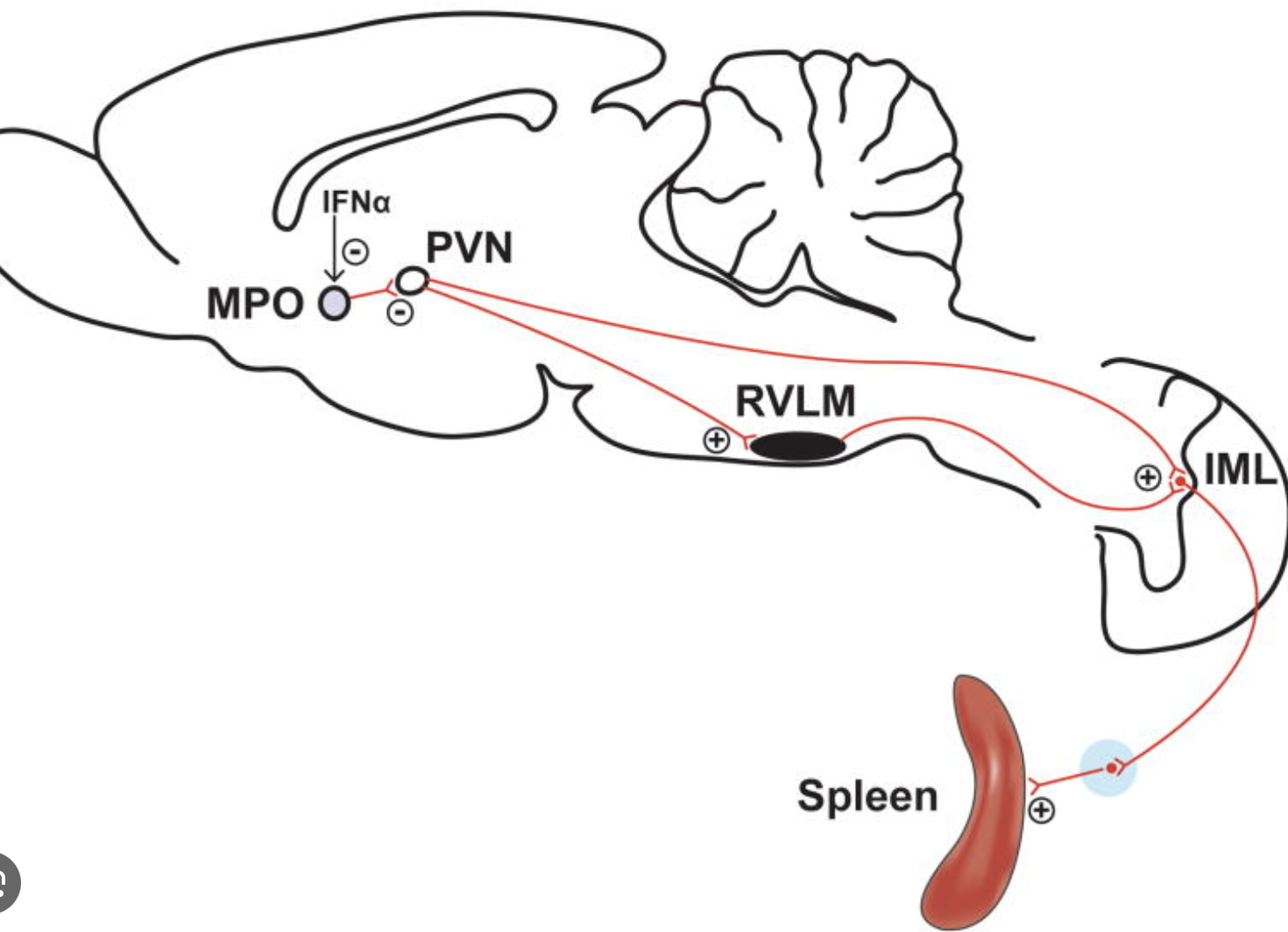
Which brain region controls SNS neurons innervating the spleen?
PVN, which is involved in the regulation of the autonomic function including the SNS.
How does septic shock cause circulatory collapse?
infection => cytokines (TNFa, IL-6)
widespread vasodilation
increased vascular permeability
as a result severe hypotension
blood also shunted to non-vital areas leaving vital organs hypoxic
heart itself may be affected / depressed
coagulation issues (microclotting aka DIC)
How do we treat the septic shock?
Septic chock is caused by cytokines e.g. IL-1, IL-6, TNF-alpha and includes severe drop in BP due to vasodilation, vascular permeability, cardiac depression; may include DIC which can include both excessive clotting and excessive bleeding as clotting factors are depleted. Treatment includes:
large amounts of IV fluids to restore blood volume
vasopressors (typically norepinephrine)
broad-spectrum antibiotics
steroids to treat refractory septic shock or adrenal insufficiency
anticoagulants (heparin) are used only on case by case basis
Why steroids?
increase sensitivity of blood vessels to catecholamines
dampen immune activation by pro-inflammatory cytokines
improve adrenal insufficiency (septic shock may cause the adrenals to produce insufficient cortisol)
Steroid dampen the immune response
steroids reduce expression of pro-inflammatory cytokines ..
.. including IL-1, IL-6, TNF-alpha, COX2 (that produces PGE2)
reduce migration of immune cells
reduce activation of T cells and macrophages
Why norepinephrine?
NE acts on a1 adrenergic receptors and has a limited action ..
.. it constricts most blood vessels and slightly increases HR
E acts on a1, b1, b2 ARs, significantly increase HR and can dilate some blood vessels
therefore NE is preferred as a more limited and controlled intervention
if NE doesn’t work, E can be used instead
Difference in the HPA / immune system in the Lewis vs Fischer rats.
Lewis rat => low HPA but highly reactive immune system => susceptible to autoimmune
Fischer => high HPA => low immune activation => no autoimmune but more infection

Is there a link between chronic stress and cancer?
Weak link.
stress => suppress immune system => cancer cells evade detection
cortisol, adrenaline => angiogenesis => helps cancer
cortisol, adrenaline => reduce apoptosis => helps cancer
stress => overeating, lack of exercise, poor sleep => cancer risk
How do cytokines (when we’re sick) affect our behaviour?
fever
slow wave sleep
social isolation
decreased eating
slow digestion
taste aversion to novel foods
hyperalgesia
Can immune cells penetrate BBB?
Yes they can. Old concept was that they can’t. Now we know they can but its just the brain rarely gets an infection. Also it was found neurons express MHC.
Wha is the role of MHC-I vs MHC-II?
MHC-I are found on all nucleated cells
MHC-II are found on dendritic and B cells, macrophages
MHC-I present intracellular pathogens (e.g. virus)
MHC-II present extracellular pathogens (bacteria, parasites)
MHC-I present pathogens to CD8 (cytotoxic T cells)
MHC-II present pathogens to CD4 (helper T cells)
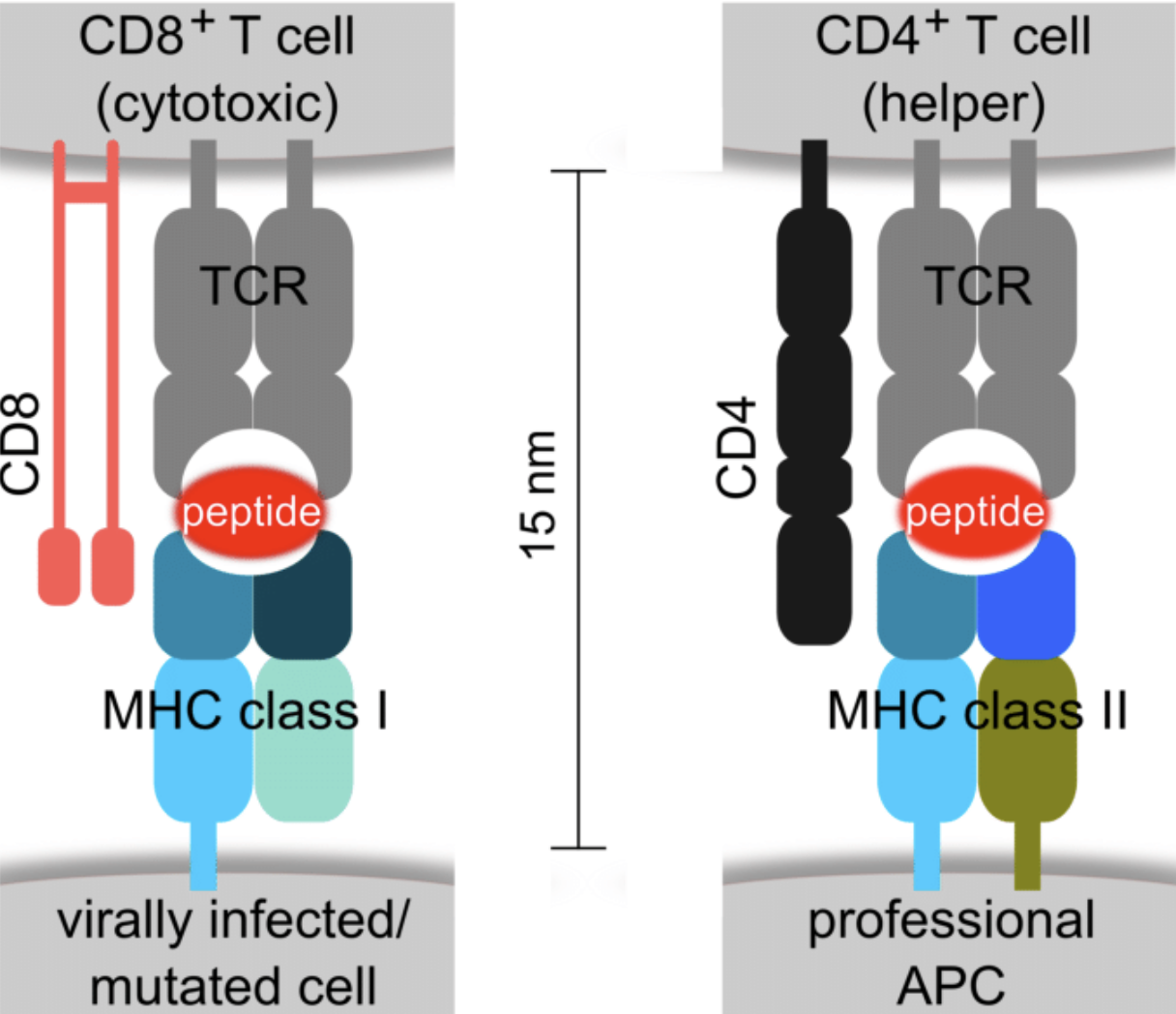
Why do neurons have MHC-I complex?
found pre- and postsynaptic at glutamate synapses
standard role of presenting intracellular pathogens to CD8 (cytotoxic T cells) and NK cells .. though immune system goes very easy on neurons to avoid inflammation
MHC-I modulate synaptic plasticity and LTP
involved in synaptic pruning (w/o pruning - autism, schizophrenia)
help during development e.g. guiding axons to their targets
interacting with glial cells
What is synaptic pruning?
selective elimination of neurons
allows to fine-tune neuronal circuits
occurs in children
w/o pruning connections are slower and noisier
w/o pruning there’s cognitive overload, difficulty focusing, slow learning
W/o pruning => autism, schizophrenia.
Explain the immune role in schizopherina.
maternal infection is risk factor for SZ
SZ associated with changes to the MHC portion on ch.6
in SZ patients acute psychosis is associated with elevated cytokines (possibly affecting the brain via circumventricular organs or vagal receptors)
How could cytokines affect the brain (esp. in schizophernia, etc)
in schizophernia elevated cytokines are accoiated with acute psychosis
possibly affecting via circumvetnroicular organs or vagal receptors
What do we need to consume to survive if we’re sick?
when we sick, our survival is prioritised
we need O2, glucose, water, salt
vitamins, minerals, amino acids are LESS important
all other things are EVEN LESS important
What is a learned taste aversion (when we’re sick)?
learned taste aversion is a classical conditioning
if we eat something and get sick (vomit, nausea, etc) we begin to associate that food with unpleasant feelings
develops with a single exposure and even if that particular food didn’t actually cause the problem
time gap can be quite long (e.g. we eat something hours before we get sick - the brain still makes a connection)
Example:
If you eat a particular dish, like seafood, and then get food poisoning a few hours later, your brain may associate the seafood with the unpleasant experience of being sick, causing you to feel nauseated or avoid seafood in the future.
How do rodents eat new food introduced to them and why?
If the food is new and unknown, they just nibble on it. This allows them to understand if it’s poisonous.
What is the “wisdom of the body” concept and why it may be wrong?
“Wisdom of the body “ concept by Curt Richter
rats given deficient diet A
then can choose b/w deficient diet A and good diet B
they choose diet B
Richter thought rats can “tell” that diet B has nutrients they needed
Rozin Experiments
Rozin showed that’s likely not true.
Experiment 1
- give deficient diet A but later inject nutrient A
- they still like diet B despite not needing nutrient A
- so perhaps they prefer B b/c it’s new and exciting?
Experiment 2
- give deficient diet A but later inject nutrient A
- choose b/w fortified diet A or deficient diet B
- they still like B - new & exciting? or perhaps A is old & boring?
Experiment 3
- give fortified A, then deficient B
- then choose b/w fortified A, deficient B and new diet C
- rats choose A (!) so it’s not about exciting new diet or boring old diet
The conclusion was that rats somehow sense which diet made them unhealthy and avoid it; at the same time they prefer the diet they’re familiar with it so long as it provides the necessary nutrients.
How does the immune system contribute to taste aversion?
nutrient deficiency leads to general malaise
causes release of cytokines IL-1, TNF-alpha
cytokines may alter food preferences; may make us more tuned to foods we’re deficient in
poor food may cause low grade gut inflammation which would cause the effect describe above
animal can also directly associate feeling sick with the usual diet and that would produce aversion
animals deficient in specific elements required by the immune system (zinc, iron) may seek those out
What are exogenous pyrogens? How do they work?
Substances that cause an increase of body temp. Could be bacterial toxins (e.g. lipopolysaccharides), pathogen-associated molecular patterns (PAMPs) e.g. flagellin, viral RNA, fungal cell wall components.
Pyrogens => macrophages/DCs/monocytes => endogenous pyrogens (e.g. IL-1, IL-6, TNF-a) => anterior hypothalamus => increases temp set point => fever => reduces pathogen survival and increases activity of the immune system and tissue repair.
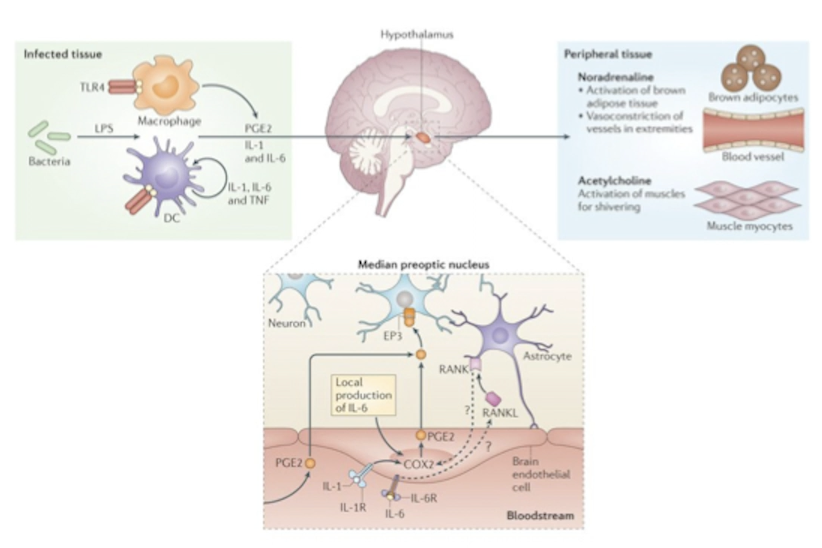
Difference b/w endogenous and exogenous pyrogens.
pyrogens increase body temperature
exogenous pyrogens are anything body recognises as a pathogen / foreign substance (bacterial toxins e.g. LPS, PAMPs e.g. flagelling, viral DBA, fungal cell wall componets
endogenous pyrogens are cytokines e.g. IL-1/6, TNF-a
exogenous pyrogens => macrophages / dendritic cells => endogenous pyrogens => anterior HT => fever
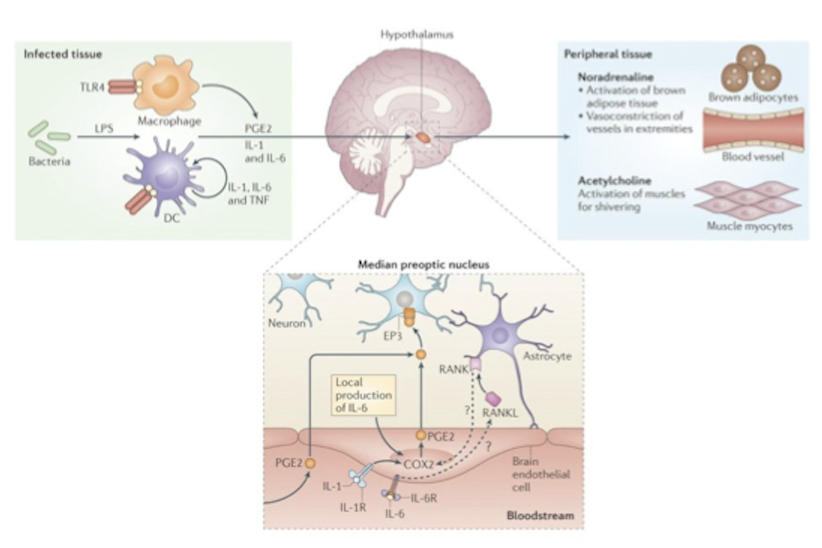
How do pyrogens produce fever and why? Explain step by step starting with pathogens entering the body.
exogenous pyrogens (LPS, flagelling, viral RNA) appear
macrophages or dendritic cells detect them
they release endogenous pyrogens (IL-1, IL-6, TNF-a)
these travel to the preoptic area of anterior HT
there endogenous pyrogens activate COX2
COX2 produces prostaglandin PGE2
PGE2 activates pyrogenic neurons
these neurons
activate brown fat
constrict blood vessels in extremities
cause rapid mucles contractions to induce shivering
this raises fever
fever makes the body inhospitable for pathogens
fever increases activity of leukocytes, speeds up tissue repair, improves production of antibodies
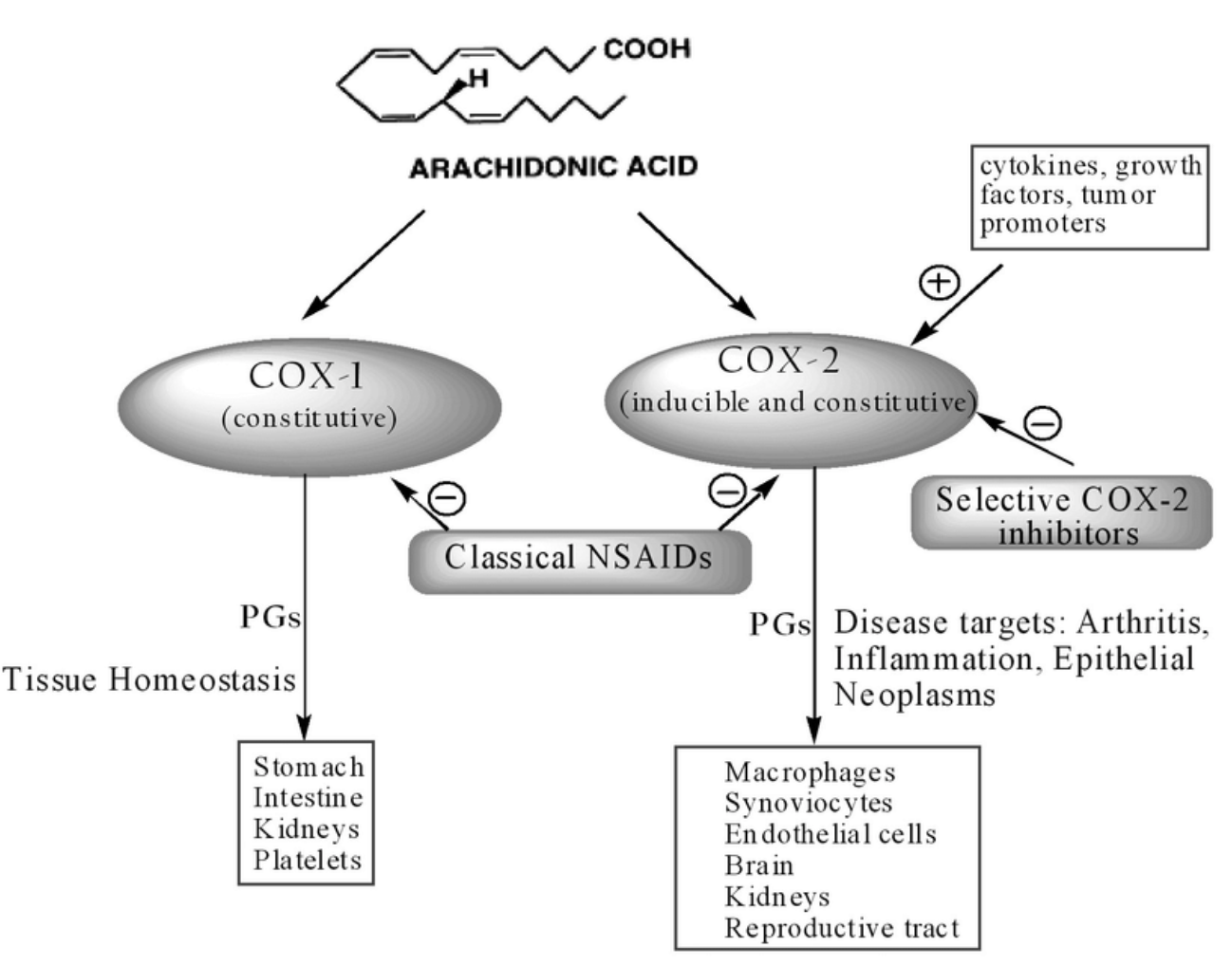
What is COX1 and COX2?
COX1/2 are cyclooxygenase enzymes. They convert arachidonic acid into thromboxane (COX1) prostaglandin PGE1/2.
COX1
COX1 is a “housekeeping” enzyme
expressed normally in many tissues
converts arachidonic acid into thromboxane-2
COX1 induces GI mucus
COX1 induces platelet aggregation to clot blood
COX1 regulate blood flow to kidneys
COX2
enzyme induced by cytokines
produced in response to infection or tissue damage
converts arachidonic acid into prostaglandin-2
in HT PGE2 induces fever
in other tissues PGE2 induces pain and inflammation
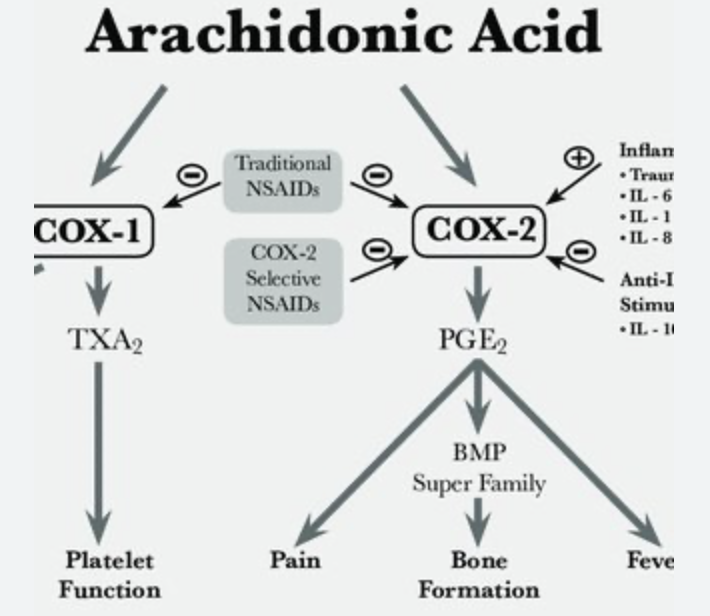
How do COX2 inhibitors work?
COX2 (cyclooxygenase) inhibitors are a type of NSAIDs
NSAIDs are non-steroidal anti-inflammatory drugs
COX2 converts arachidonic acid into prostaglandin PGE2
PGE2 activate vlPOA in the HT to induce fever
PGE2 in other tissues induce pain and inflammation
COX2 inhibitors prevent that
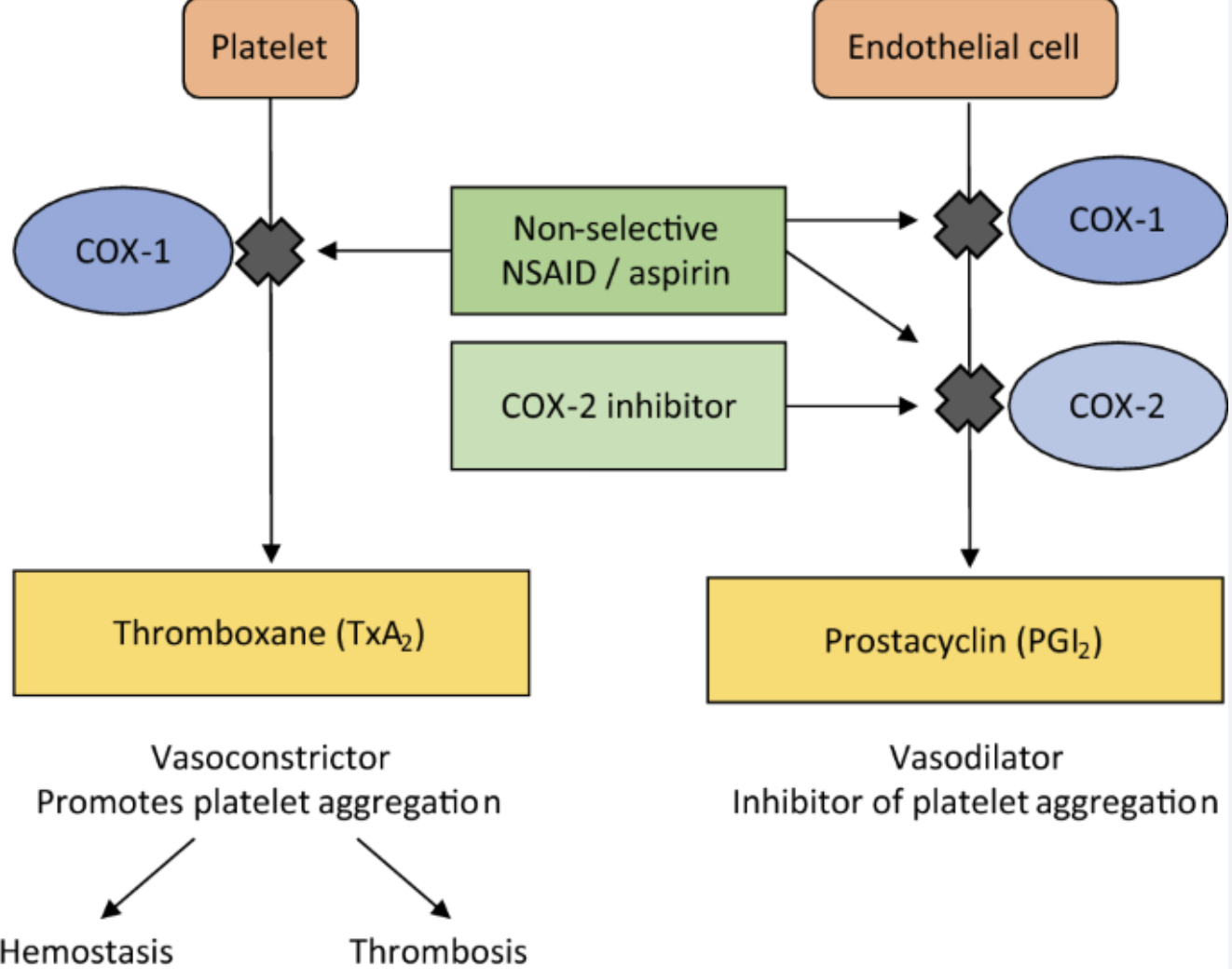
What are COX inhibitors? Difference b/w COX1 and COX2 inhibitors.
COX inhibitors inhibit cyclooxygenase
COX1 is required for normal “housekeeping” (kidney function, blood clotting, GI tract mucus / protection)
COX2 is induced in response to infection / inflammation
COX inhibitors are non-selective NSAIDs (non-steroidal anti-infloammatory drugs)
called non-selective b/c suppress both COX1 and COX2
aspirin, ibuprofen, acetaminophen, diclofenac
reduce fever, inflammation, pain
aspirin also reduces blood clotting
side effects: GI bleeding, stomach ulcers, gastritis, risk of bleeding
there are COX2-specific inhibitors e.g. celecoxib
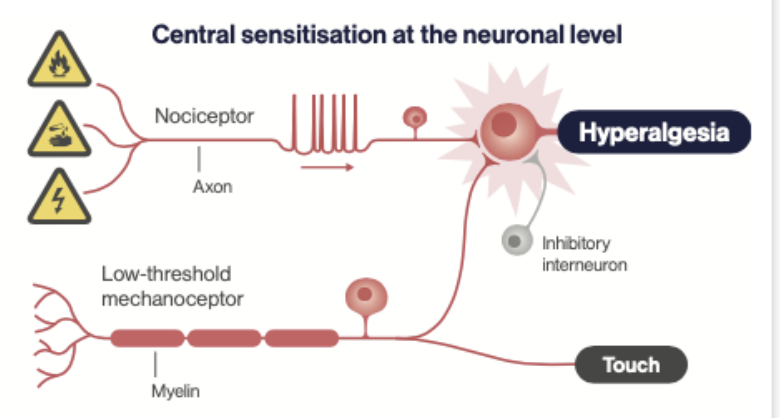
Why does infection promote hyperalgesia?
To promote social isolation, inactivity, care of sites of injury, to reduce energy use and promote recuperation.
How doe infection promote hyperalgesia?
Brain
Infection => IL-1 and TNF-alpha => activates CN X => NTS => Raphe => Dorsal Horn. This enhances pain transmission.
Tissue
lowers activation threshold of nociceptors (via PGE2)
activates nociceptors directly (substance P)
What is primary and secondary hyperalgesia in response to infection?
infection activates the immune system (IL-1, IL-6, TNF-a)
these stimulate PGE2 in tissues and brain
primary hyperalgesia: due to peripheral sensitisation by PGE2 when pain threshold of nociceptors is lowered
secondary hyperalgesia: due to central sensitisation (as pictured) in the spinal cord and brain
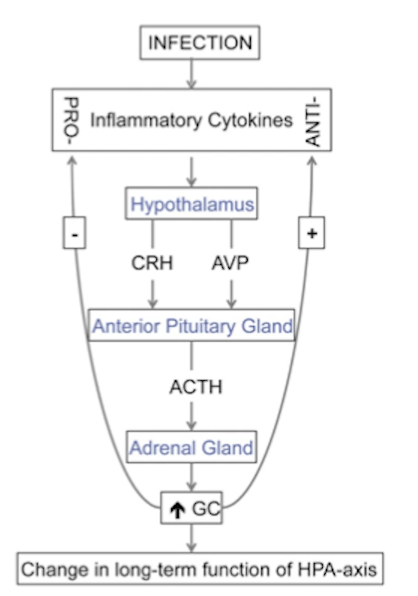
Which three cytokines activate the HPA axis?
IL-1, IL-6, TNF-alpha.
IL-1 stimulates both CRH and ACTH.
IL-6 also stimulates CRH neurons to release ADH causing hyponatremia.

What are the synergistic abilities of IL-6 when it acts on CRH neurons? What role does ADH play?
Il-6 / CRH neuron synergy is critical for stress response to inflammation.
during immune response immune cells produce IL-6
IL-6 cross the BBB and actives CRH neurons in the PVN
IL-6 not only activates them but also enhances their sensitivity
this creates a +’ve feedback loop responding with stress to inflammation
Role of ADH:
CRH neurons co-release ADH as well
ADH enhances action of CRH on production of ACTH
note that IL-6 (and other cytokines) cause vasodilation
ADH can counteract that by constricting blood vessels systemically (restoring BP) while at the site of infection IL-6 will keep blood vessels dilated
Note that normally AVP is produced by magnocellular neurons in the PVN in response to the stimulation by neuronas in the circumventricular organs e.g. subfornical organ.
Problem with using male rats in studying mental health.
Sex differences seem to influence mental health and most other aspects of the neuro/endocrine systems.
E.g. in the US / Europe / Asia women are at a higher risk of developing Alzheimers.
Sex differences in hippocampus. What causes these differences?
men: better at spatial navigation
women: better verbal memory and social perception
hippocampus shows stronger activation in women during memory tasks
also stronger connectivity b/w hippocampus and amygdala in women
Differences likely influenced by estradiol b/c it promotes neurogenesis, dendrites growth and spines, as well as hippocampal volume.
Why does hippocampus makes its own estrogen?
Hippocampus has very high neural plasticity and this is aided by estrogen. Estrogen also protects neurons from damage e.g. ROS, inflammation.
Which hormone affects the onset of Alzheimer’s?
Lack of estrogen increases the risk. Low estrogen increases systemic inflammation. Inflammation contributes to Alzheimers.
What explains the fact that women are more at risk of Alzheimers.
it’s not known what causes Alzheimer’s
one hypothesis is that it’s due to neuroinflammation
women have stronger immune system response
estrogen is neuroprotective and anti-inflammatory and therefore post-menopausal women are at an especially high risk
while men have lower estrogen than women of any age; men benefit of testosterone aromatised to estrogen
women live longer than men
these factors explain why women are at higher risk for Alzheimers than men
Are symptoms of Alzheimers worse in men or women?
The symptoms of A’s are worse in women. Semantic memory is worse in women.
How does estrogen replacement therapy affect Alzheimer’s?
One study showed ERT sped up the onset of AD. However in this study they gave ERT to women in their 60s, so 15 years after the drop in estrogen. So possible causes:
brain already adapted to low E and ERT disrupted this
artificial E is different from real E
they may have used progestins which can be damaged
Other studies showed beneficial effects of ERT especially if started early.
Autism has heritability of about 70%. If 100 couples where one of parents have autism have one child (i.e. 100 children) how many children will have autism?
Impossible to say. Heritability in this case is how much autism can be explained by genetics (in this case 70% by genetics, 30% by other factors e.g. environment).
Imagine if ASD had 0% heritability. We can’t say that just because it has heriability of 0%, then 100 autistic parents will produce 0 autistic children - other factors (environment, etc) may still cause a child to develop autism.
Is autism heritable?
ASD is highly heritable
90% concordance in MZ twins
heritability in 55-80%
polygenetic condition
parents tend to be emotionally aloof and/or scientists
Is there drug treatment of autism?
No.
anti-psychotic drugs may reduce aggressive behaviour
antidepressants and anti-ADHD drugs don’t help
What does that mean that autistic children have impaired social cognition / theory of mind? How can we test this?
They don’t understand what other people experience, can’t put themselves in their shoes. Simple test:
tell a child being tested a story
in a story there are two kids in a room
one kid puts a toy in a basket and leaves
the other kid moves the toy behind the curtain
the first kid returns and starts looking for the toy
Ask your child where would the kid look? If the child has a theory of mind, they will understand that the first kid will first look in the basket, because this kid wasn’t in the room when the toy was moved.
How are patterns of neuronal activation different in autism?
cerebellum
striatum
amygdala
hippocampus
insular cortex
They have reduced activation in social cognition areas (PFC, tmpoeral-parietal junction, fusiform face area)
What
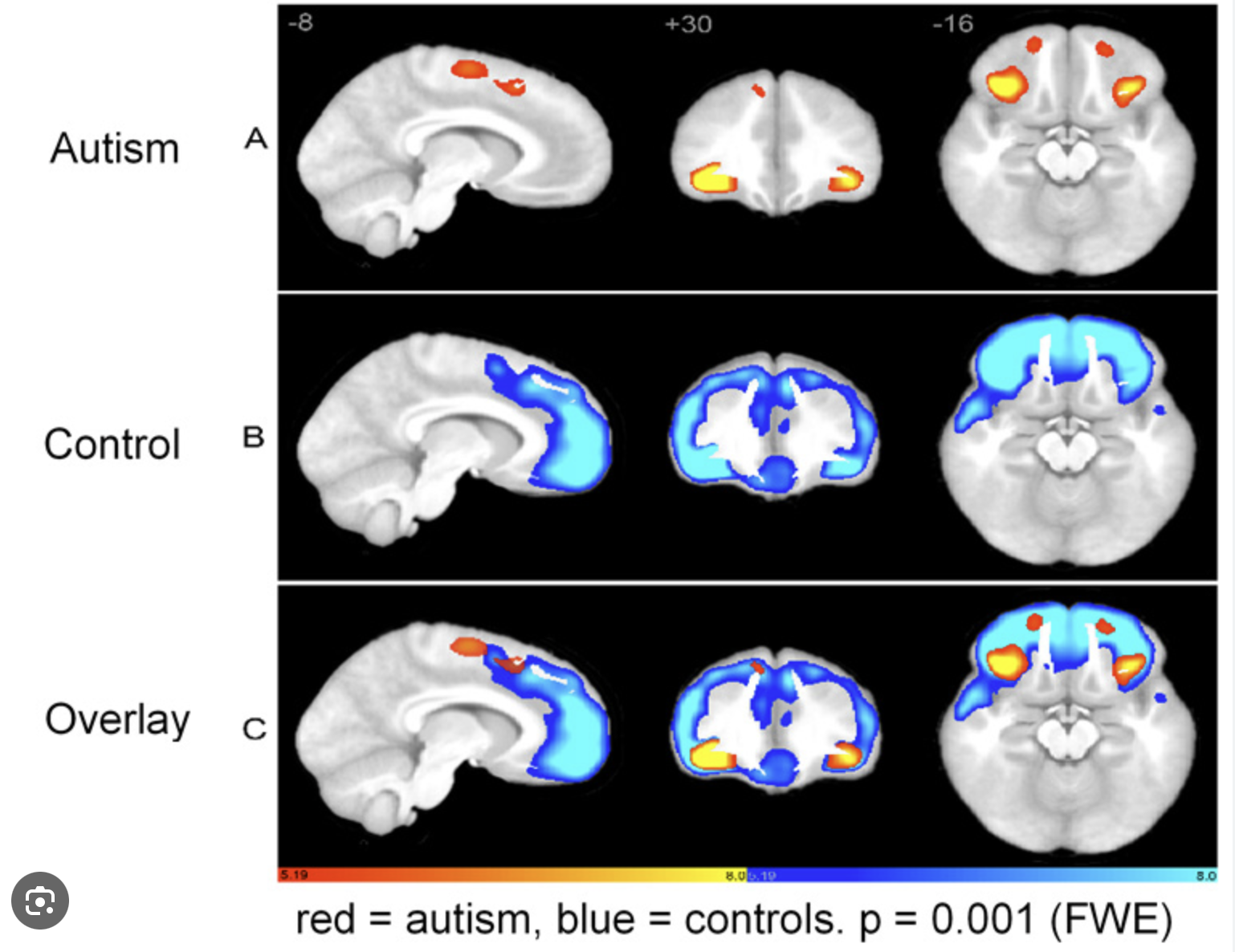
How are brains of autistic people different?
abnormal head growth (it’s larger early in development)
maaaybe low IGF-1 (needed to cerebellar growth)
early brain overgrowth; poor pruning => overstimulation
reduced connectivity b/w brain region (white matter)
PFC, amygdala, temporal lobe, cerebellum, corpus callosum are different
What are approaches to study autism in animal models?
find animals with social avoidance (but doesn’t truly mimic autism)
find animals that mimic Rett or fragile X syndrome in humans (both have typical autistic symptoms)
Rett => girls, child regresses, loses motor skills, ability to speak, anxiety
Fragile X => boys, intellectual disability, anxiety, long face, prominent jaw, big ears
Connect oxytocin and autism.
OTR knockout mice have impaired social memory
intranasal oxytocin enhances trust in NT individual
OTR expressed in frontal cortex, olfactory nucleus, amygdala, hypothalamus
individual with overexpressed OTR show generous behaviour, are pro-social, show empathy, less stressed, are better parents
some children with autism have lower levels of OT
there’s increased rate of mutation in OTR
intranasal OT improved autistic children’s emotion recognition, recognition of social cooperation, preference and time gazing at pictures of faces
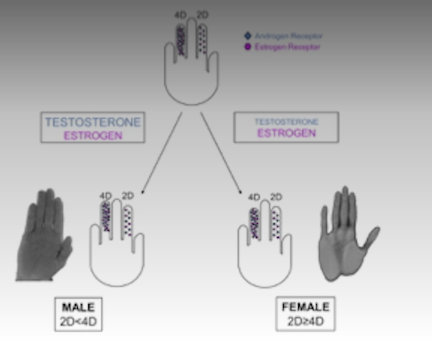
Sexual dimorphism of autism?
More common in males.
“Extreme male brain” theory - more systematic, less empathetic brain.
There seem to be androgen receptor mutation in females with autism.
Digit ratio suggest excess T exposure in autism.
Describe cognitive dysfunction in T2DM.
Reduced:
- intelligence
- psychomotor efficiency
- cognitive flexibility
- processing speed
- exectuvie function
- memory
Diabetes increases the risk of demetia by 19%
Atrophy of white matter.
Specific brain regions affected in T2DM.
hippocampus: reduced contextual memory
NAcc: explains reduced reward responsiveness
PFC: loss of cognitive control
Amygdala: high anxiety, less social affiliation
Improved BGL does not reverse these changes, but GLP-1 improves the brain. Intranasal insulin improves hippocampus.
What does higher IGF-1 do in aged rats?
IGF-1 seems to improve hippocampus and related memory tasks; improves denritic branching. Perhaps indicative of T2DM related decline in hippocammpus. Perhpas insulin resistance in the brain contributes to cognitive decline.
Is insulin resistance in the brain possible?
T2DM is known for various cognitive deficienices (hippocampus -memory; NAcc - anhedonia; amygdala - anxiety; PFC - poor cognitive control).
Insulin receptors are found in many areas of the brain. Insulin modulates LTP. While neurons take up glucose in insulin-independent manner, astrocytes and oligodendrocytes require insulin - so they may be vulnerable.
What is migraine?
headache disorder
moderate to severe headaches
throbbing and pulsating
last hours to days
can also have nausea, photophobia, phonophobia
neurological condition
Phases include prodrome, aura (some ppl), headache, postdrome.
What triggers migraine?
hormonal changes esp. female cycles
stress
certain foods
caffeine, alcohol
sleep deprivation
weather changes
bright lights, loud noises, strong smells
dehydration or fasting
Prevalence of migraines.
15% of population
typically ages 22 to 55
most common debilitating brain disorder
3:1 female to male
runs in families
Mechanisms of migraine.
Cortical spreading depression
Wave of electrical activity; followed by neuronal inhibition. This triggers changes in NT and inflammatory substances. This wave activates trigeminal nerve system (leading to headache) and sometimes aura.
Trigeminal vascular system
Trigeminal nerve activated => releases calcitonin gene-related peptide (CGRP), substance P => vasodilation and inflammation of meningeal blood vessels => trigeminal ganglion carries pain signal => brainstem and HT / reward system.
In what condition do we find cortical spreading depression? Why does it happen?
Migraine is preceded by cortical spreading depression. Wave of electrical activity; followed by neuronal inhibition. This triggers changes in NT and inflammatory substances. This wave activates trigeminal nerve system (leading to headache) and sometimes aura.
It happens due to predispoision to neuronal hyperacitity, possibly due to mutations in Ca channels, Na/K ATPase, VG-Na channels, glutamate related proteins, etc.
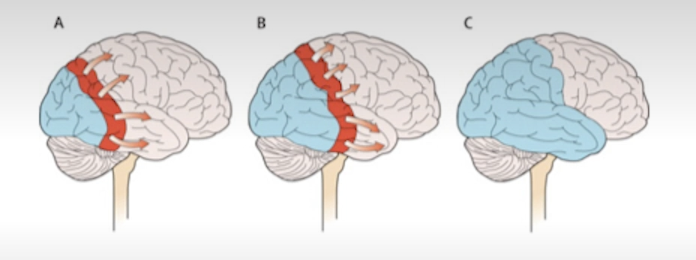
What changes does migraine cause in the brain, structurally?
thicker somatosensory cortex
more gray matter in caudate
less gray matter in amygdala and various cortical areas
Though these changes may be related to pain as a symptom, rather than migraine itself.
Novel tx for migraine.
CGRP (calcitonin gene-related peptide) antibodies (one injection lasts months)
vagal nerve stimulation
anticonvulsants
botox (reasons not understood; maybe less signalling via trigeminal nerve)