OCR A 6.2.4 Carbon Carbon Bond Formation
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
extending C chain
-uses C-C bond formation
-carbon chains cant be added tgthr in their simple hydrocarbon form
-issue as extending C chain to synthesis organic products is important
-we use reactants and reagents that have C atom which acts as nucleophile/electrophile
cyanide
-negatively charged atom
-nucleophile
cyanide w/ haloalkane
-halogen more electronegative than C; pull e towards themselves in covalent bond; polar bond
-C ca be attached by cyanide nucleophile
-extend C chain as -CN substitutes halogen
-produces C-C bond between d+ C and C on cyanide
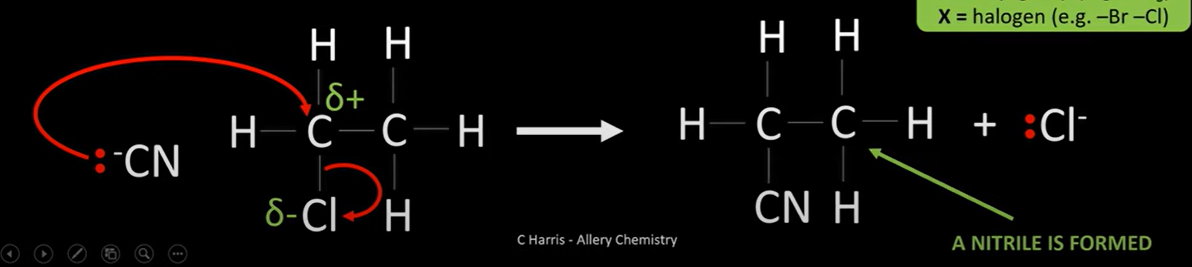
haloalkane reaction w/ cyanide
-make nitrile
-nucleophilic substitution
-conditions; warm ethanoic potassium cyanide, under reflux
-nucleophile attacks d+ C and replaced halogen
-C-X breaks, both e move from bond to halogen
-new bond formed between CN ion and C
cyanide mechanism reaction w/ ethane
KCN reaction w/ carbonyl compound
-produces hydroxynitrile
-mixture of KCN and H2SO4 used
-nucleophilic addition
-CN- ion attacks d+ C; lone pair of e donated from CN ion
-2 e in dbl bond transferred to O
-as KCN is acidified there is supply of H+ ion in sol
-H+ ion accept lone pair of e from O
why HCN not used
-too poisonous
how to make amine
-reduce nitrile using H2 gas and Ni/Pt catalyst used, w/ high temp and pressure

hydrolysis of nitriles
-form carboxylic acids by heating w/ dilute aq acid (HCl)

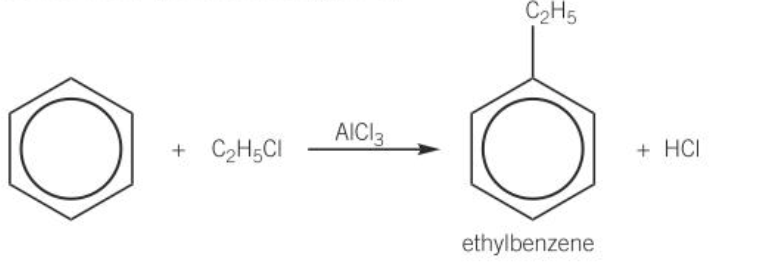
alkylation
-reaction that transfers an alkyl group from haloalkane to benzene ring
-AlCl3 catalyst
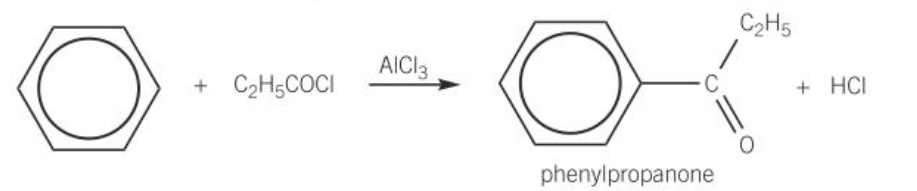
acylation
-when benzene reacts w/ acyl chloride to form ketone
-AlCl3 catalyst