matter
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
pure substances are…
uniform throughout
two types of pure substances:
elements
compounds
element:
only one type of atom
CANNOT be broken down at all
monatomic;
exist as single atoms
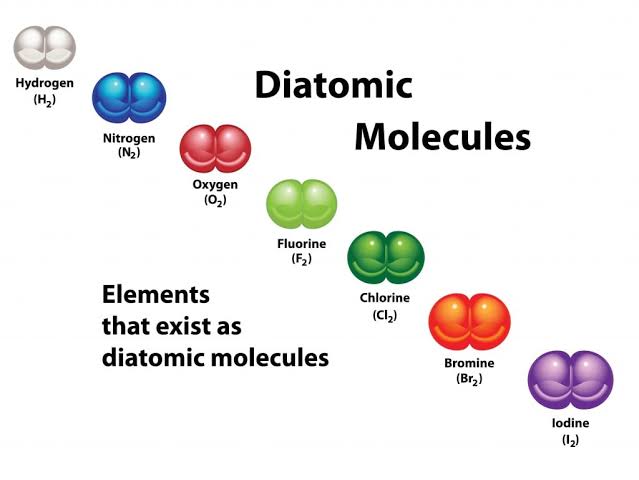
diatomic:
exists as molecule
2 of same atom
diatomic elements:
Br
I
N
Cl
H
O
F
molecule:
more than one atom
compounds:
2 or more different atoms
can be CHEMICALLY broken/seperated
WHOLE NUMBER RATIOS
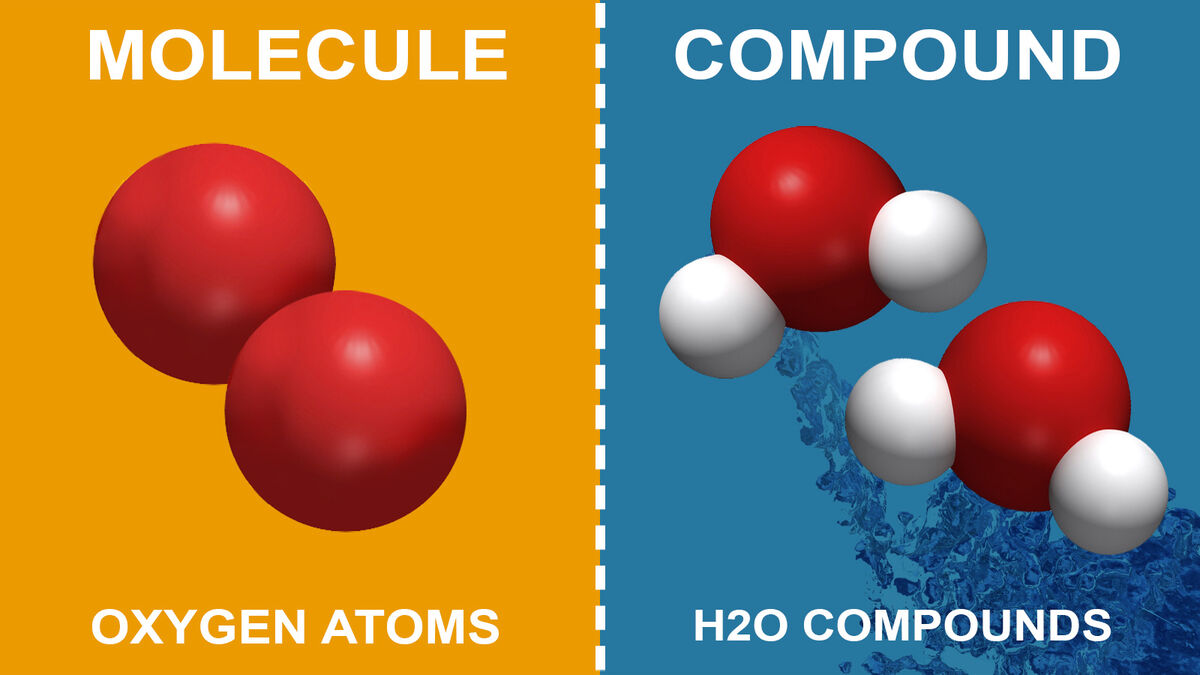
examples of compounds:
H2o
NaCl
the two types of mixtures…
homogeneous and heterogeneous
homogeneous
EVENLY/UNIFORMLY mixed
solutions (aq)
CAN BE seperated by EVAPORATION, DISTILLATION
CANNOT be seperated by FILTRATION
heterogeneous
UNEVENLY mixed
you can see diff parts
ex. SAND + WATER
CAN BE seperated by DISTILLATION, EVAPORATION + FILTRATION
(aq) = ?
dissolved
filtration
seperated solid from liquid
separation of heterogenous mixtures
CANNOT BE USED to separate homogeneous