Chem 115 - Exam 3 Orgo, Structures, Polymers
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
polarity
difference in electronegativity
covalent bonding
2 non metals
similar electronegativity
e- shared
ionic bonding
metal and nonmetal
different electronegativity
e- fully taken by more electronegative
metallic bonding
2 metals
conductive
electron group arrangements
linear
trigonal planar
tetrahedral
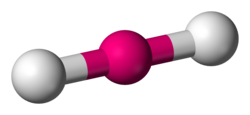
name this structure and its angles
linear
180
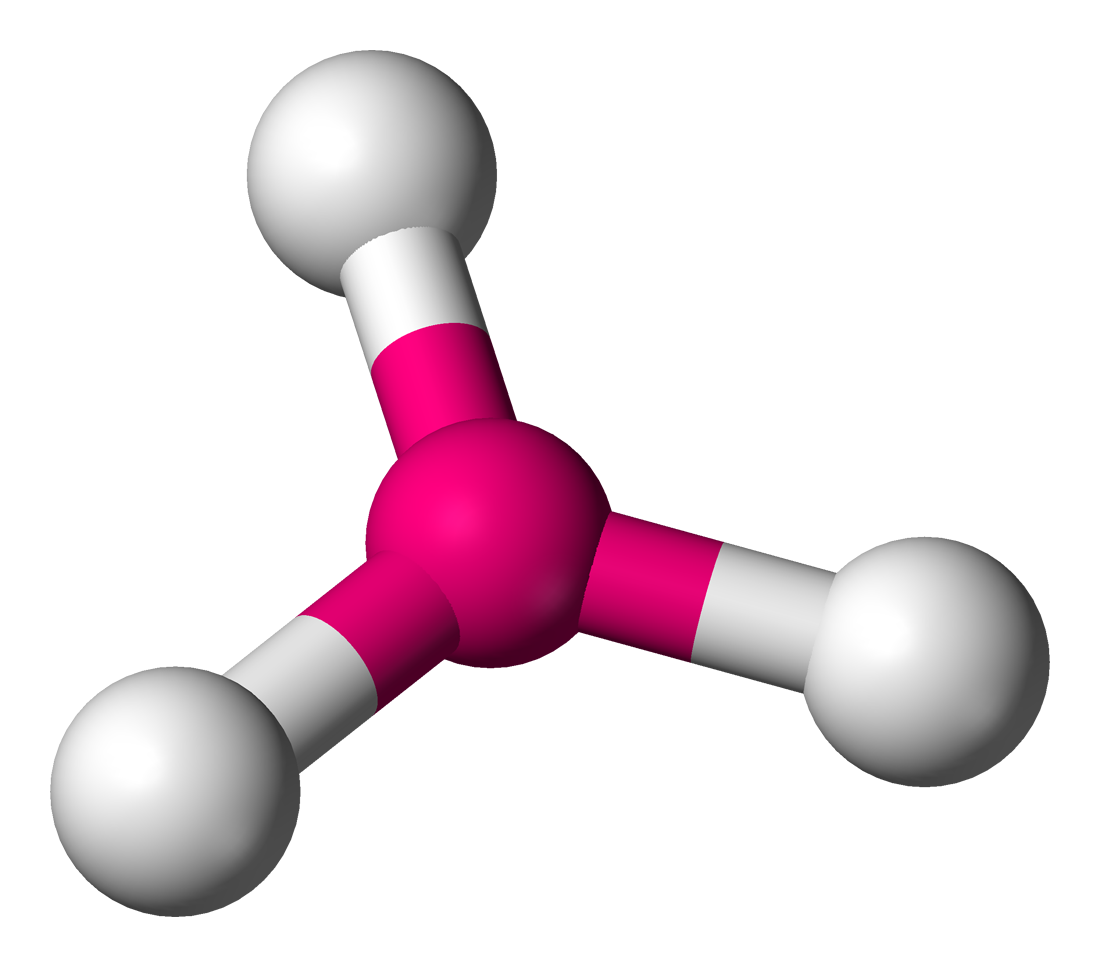
name this structure and its angles
trigonal planar
120
name this structure and its angles
bent
< 120
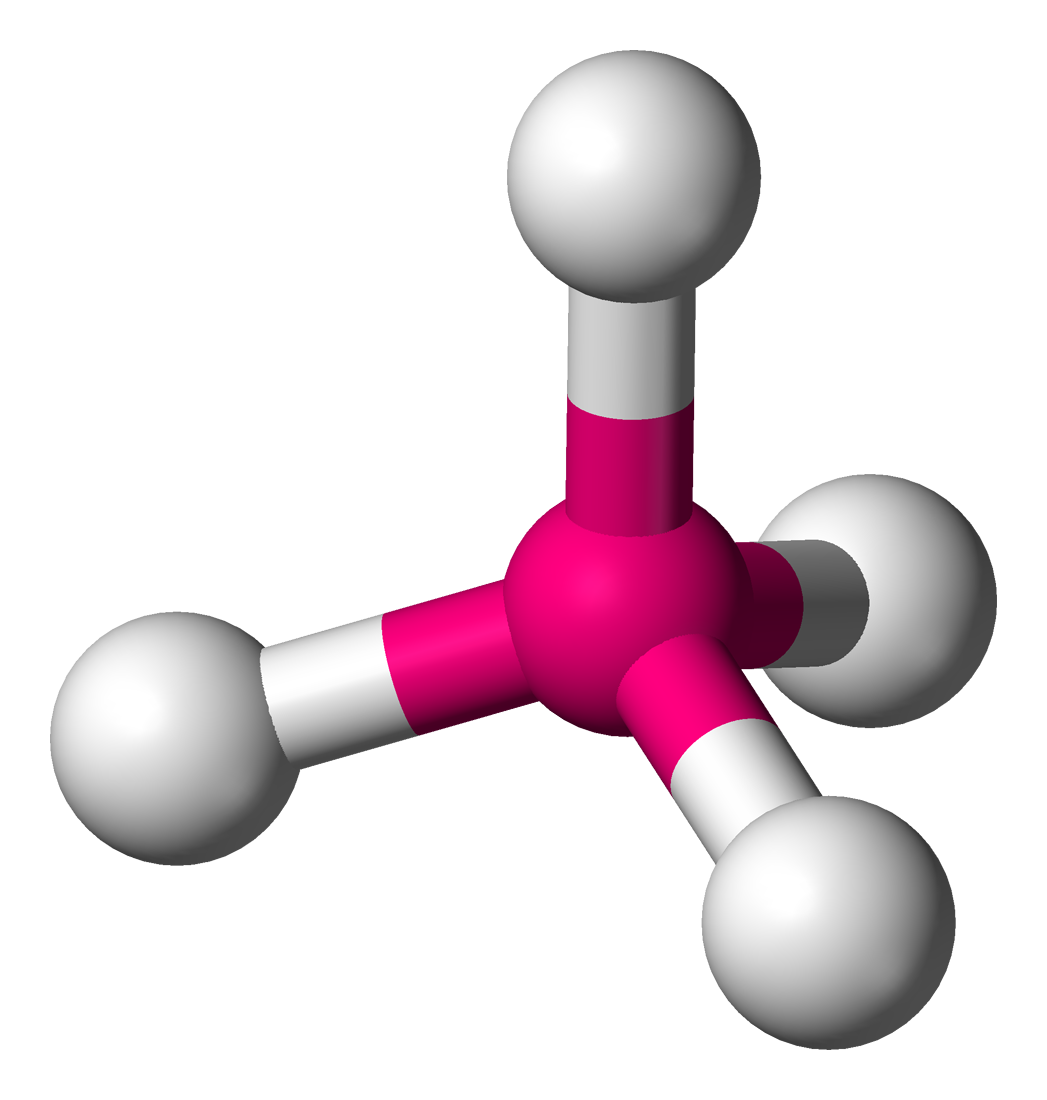
name this structure and its angles
tetrahedral
109.5
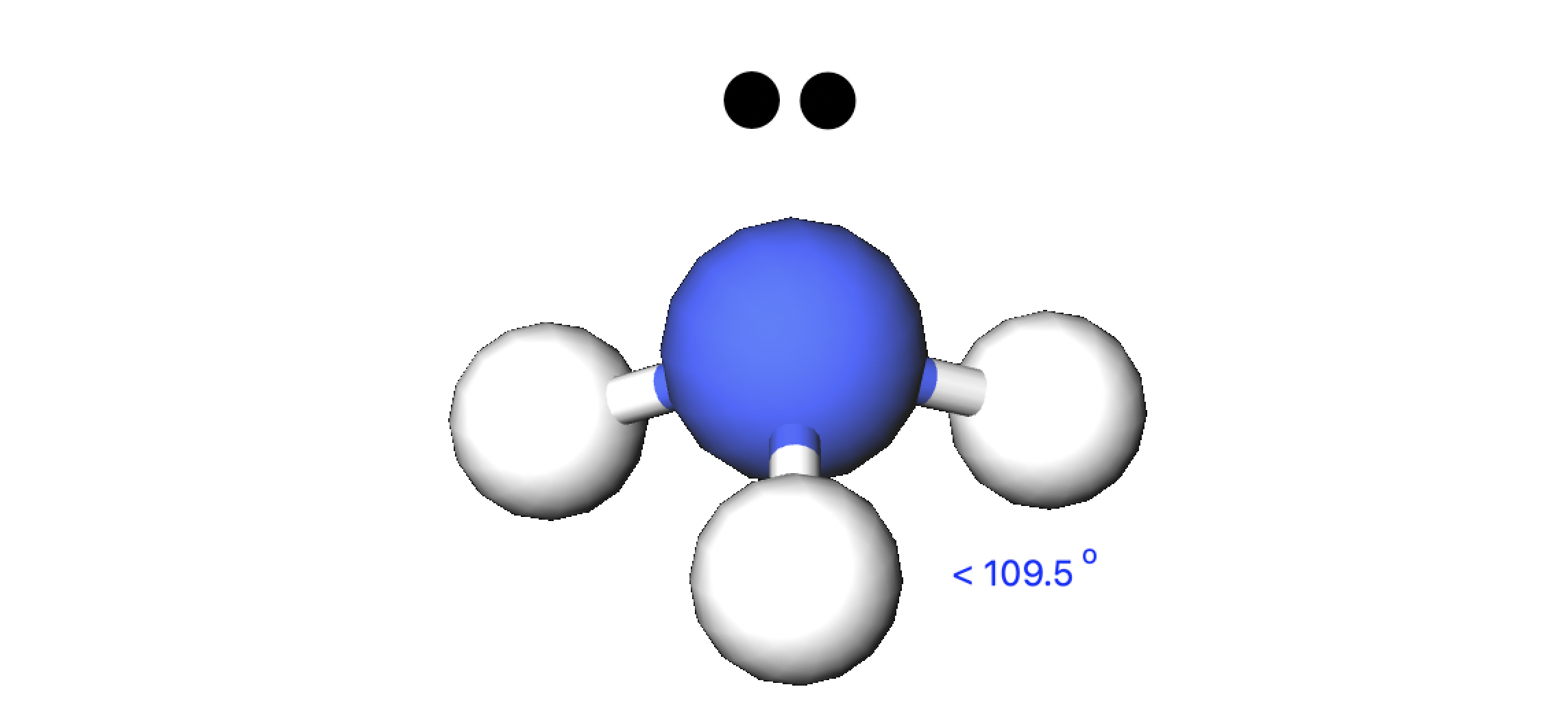
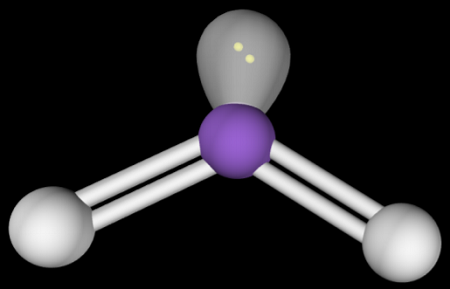
name this structure and its angles
trigonal pyramidal
<109.5

name this structure and its angles
bent
< 109.5
formal charge formula
valency - (#sticks around atom + #dots around atom)
when can elements overfill their octet?
when they’re past phosphorus
single bonds
1 sigma
weak
free rotation
double bonds
1 sigma 1 pi
no free rotation
triple bonds
1 sigma 2 pi
no free rotation
strong
prefix for 1
meth-
prefix for 2
eth-
prefix for 3
prop-
prefix for 4
but-
prefix for 5
pent-
prefix for 6
hex-
prefix for 7
hept-
prefix for 8
oct-
prefix for 9
non-
prefix for 10
dec-
prefix for ring
cyclo-
ending for single bond
-ane
ending for double bond
-ene
ending for triple bond
-yne
naming a chain with subunit
lead with carbon number placement
ir
higher frequency → faster vibration → stronger bond
hydrogen bonding
hydrogen with
nitrogen
oxygen
fluorine
im forces (strong → weak)
ion - dipole (ion + polar)
hydrogen bond
dipole - dipole (polar + polar)
ion - induced dipole (ion + nonpolar)
dipole - induced dipole (polar + nonpolar)
dispersion
hybridization
2 electron groups: sp
3 electron groups: sp2
4 electron groups: sp3
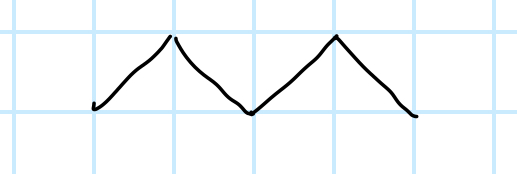
name the functional group
alkane
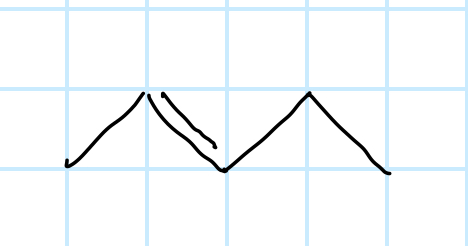
name the functional group
alkene

name the functional group
alkyne
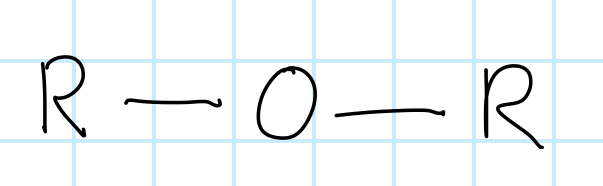
name the functional group
ether
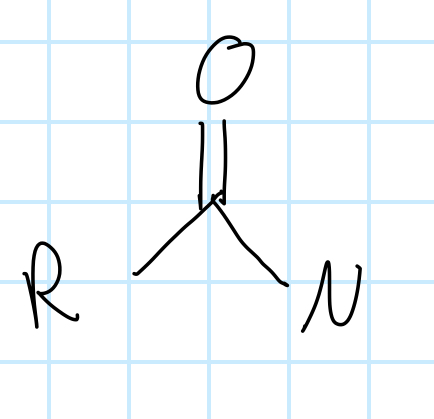
name the functional group
amide
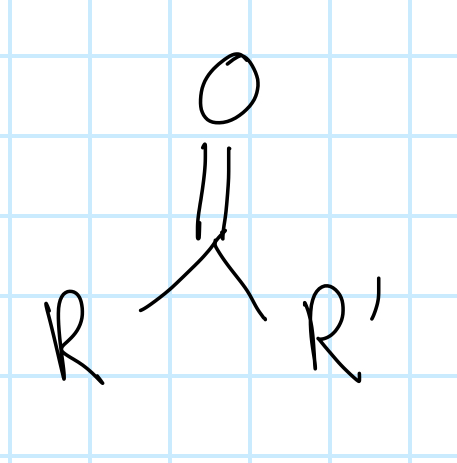
name the functional group
ketone
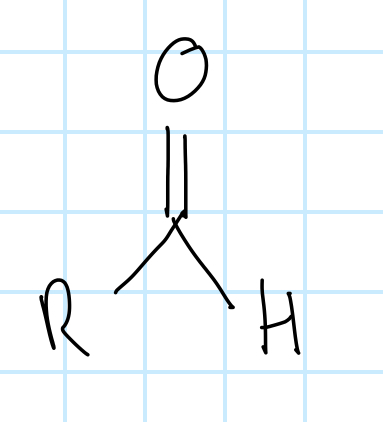
name the functional group
aldehyde
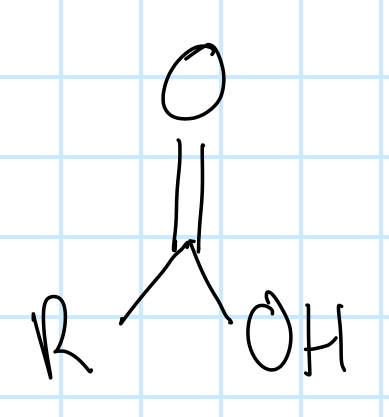
name the functional group
carboxylic acid
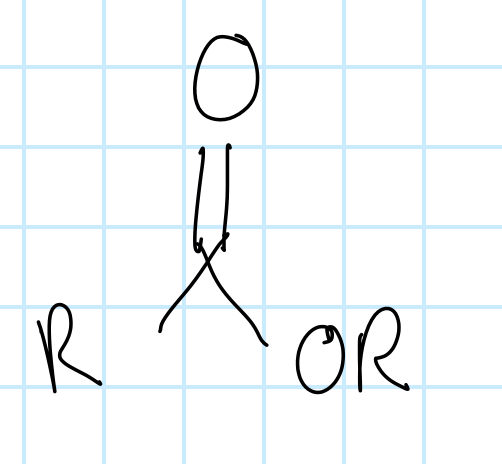
name the functional group
ester
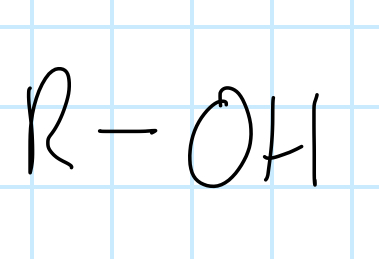
name the functional group
alcohol