Core Practicals Chemistry
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Last updated 2:36 PM on 4/7/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards
Core Practical 1 - measure the molar volume of a gas
find volume of CO2 produced when you react acid and a carbonate
2
New cards
CP1 - what reagents
calcium carbonate (CaCO3) and ethanoic acid
3
New cards
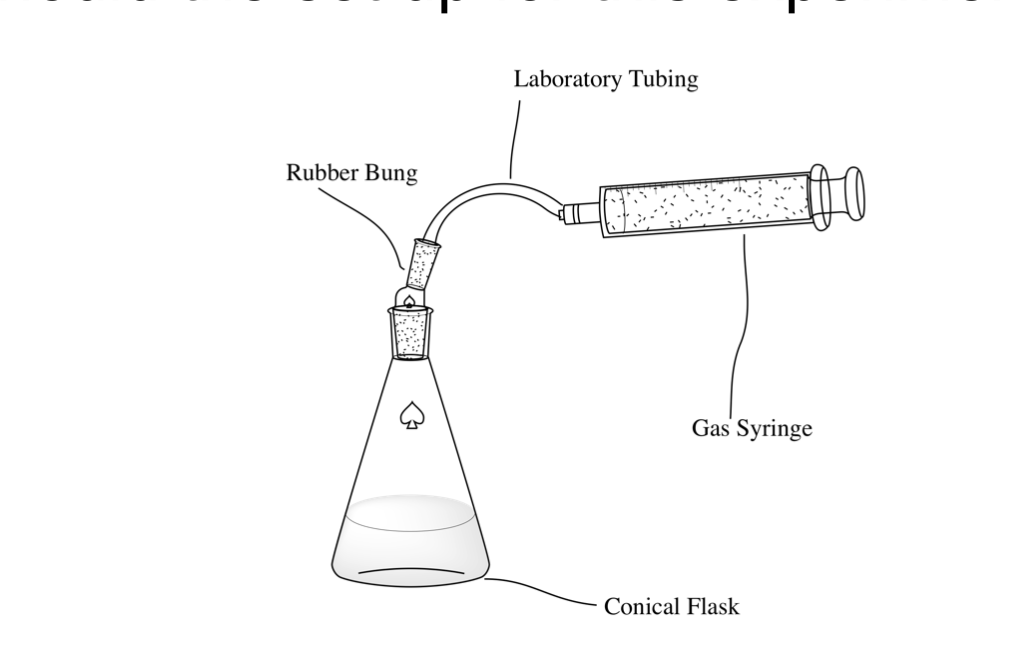
CP1 - equipment required
gas syringe or upturned measuring cylinder
conical flask
bung
conical flask
bung
4
New cards
CP1 - data analysis
vary mass of CaCO3, plot a graph
1:1 ratio so find moles of CO2 compared to volume
scale up to 1 mol
should be around 24
1:1 ratio so find moles of CO2 compared to volume
scale up to 1 mol
should be around 24
5
New cards
CP1 - key points
weigh by difference
reduce escaping gas by putting CaCO3 in a small container in the flask and tipping to combine
reduce escaping gas by putting CaCO3 in a small container in the flask and tipping to combine
6
New cards
CP1 - issues
CO2 is slightly soluble in water, some might dissolve
escaping gas
escaping gas
7
New cards
Core practical 2 - prepare a standard solution from a solid acid and use it to find the concentration of a solution of sodium hydroxide.
prepare a standard solution of sulfamic acid, then titrate with the NaOH
8
New cards
CP2 reagents
sulfamic acid NH2SO3H solid
dilute with water
NaOH solution of unknown concentration
dilute with water
NaOH solution of unknown concentration
9
New cards
CP2: steps
1. weigh by difference, find mass of sulfamic acid
2. add to volumetric flask, rinse everything it touches into the flask
3. fill with water up to the line (meniscus)
4. cap on and shake
5. put this into the burette and titrate with the NaOH
6. find the mean titre
7. find mols of sulfamic acid required
8. 1:1 ratio - mols of NaOH in solution - conc
10
New cards
Core practical 3 - find the concentration of a solution of hydrochloric acid
titrate against NaOH
1. rinse everything with H2O then reactant
2. use pipette for NaOH (known concentration) and transfer to flask
3. burette filled, normally with acid
4. add some titrant, swirl, read titre
5. repeat until concordant
6. do a rough titre first and don’t include in concordance
7. calculate mean titre
1. rinse everything with H2O then reactant
2. use pipette for NaOH (known concentration) and transfer to flask
3. burette filled, normally with acid
4. add some titrant, swirl, read titre
5. repeat until concordant
6. do a rough titre first and don’t include in concordance
7. calculate mean titre