Concept 3.3: Acidic and basic conditions affect living organisms
1/8
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
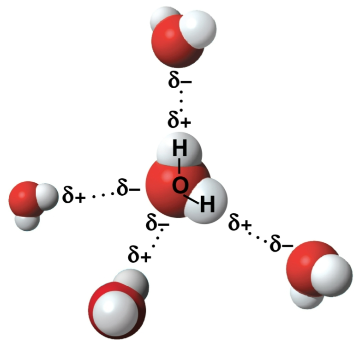
Hydrogen bonds
Bonds between hydrogen atoms and oxygen atoms of different water molecules, creating hydrogen ions (protons) (H+) that result in one hydroxide ion (OH-) and one hydronium ion (H3O+)
Hydroxide
A water molecule that has lost a proton / hydrogen ion due to hydrogen bonding, represented as OH-
Hydronium
A water molecule that has gained an extra proton / hydrogen ion due to hydrogen bonding, represented as H3O+ or simply H+
Hydrogen ion
A shifting hydrogen atom between two water molecules in a hydrogen bond
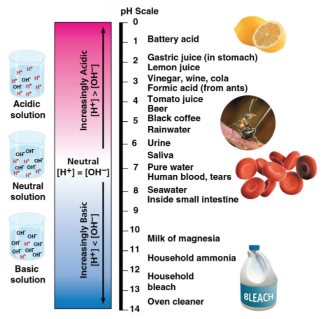
pH scale
A measure between the concentrations of H+ and OH- due to the presences of acids and bases
Acid
A substance that increases the H+ and reduces the OH- concentration of a solution with a pH less than 7
Base
A substance that increases the OH- and reduces the H+ concentration of a solution with a pH greater than 7
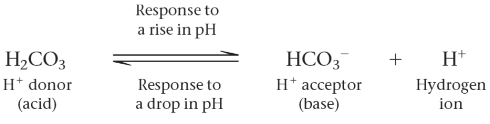
Buffer
Substances that minimize changes in the concentrations of H+ and OH- of a solution to ensure pH levels closer to neutrality (7) using a weak acid and weak base
Ocean acidification
The formation of carbonic acid in seawater due to the presence of carbon dioxide, potentially harming underwater organisms by weakening shell development and coral formation by inhibiting calcification