Chemistry - The Mole Concept + Redox Reactions + Kinetic Theory
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
mole
the amount of a specific substance that is proportionate to the amount of elementary entities present in 12g of carbon-12
Describe Avogadro’s number and Avogadro’s Law and its postulates
The amount of elementary entities present in 12g of carbon-12 is represented as 6.022 × 10²³ or Avogadro’s number
Avogadro’s Law states that equal volumes of all gases at the same temperature and pressure have equal numbers of molecules
At room temperature and pressure, 1 mole of any gas occupies 24.0dm³.
At standard temperature and pressure, 1 mole of any gas occupies 22.4 dm³.
Describe molar mass and its formula
the mass of one mole of a specific substance in grams
mass of substance/molar mass
Differentiate between the empirical formula and molecular formula
empirical formula - simplest whole number ratio of elements present in a compound/molecule
molecular formula - total number of each atom present in a molecule
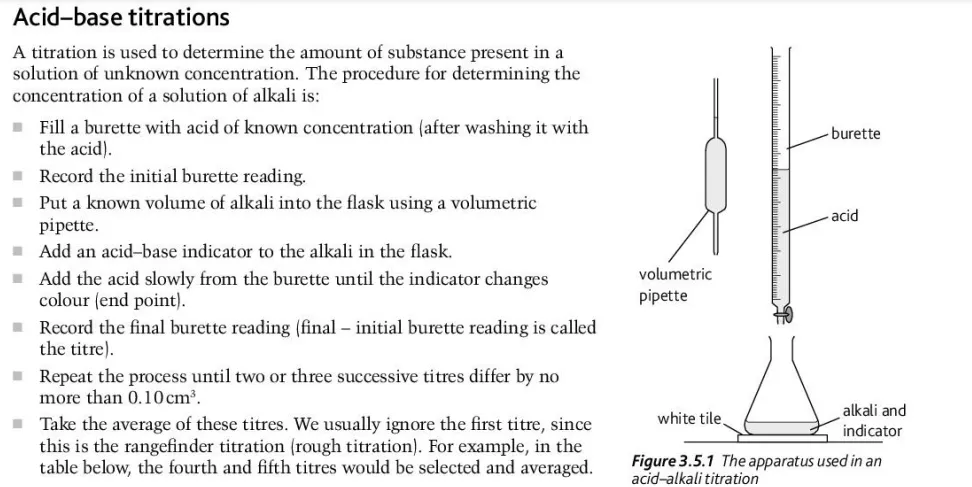
What are the steps for an acid-base titration?
redox reaction
a reaction in which one element loses electrons (reduction) and another gains electrons (oxidation)
half equation
a chemical equation which shows either the reduction half or the oxidation half of a redox reaction
State the reactivity series of metals
The reactivity series of metals include K, Na, Ca, Mg, Al, Zn, Fe, Pb, H, Cu, Ag, Au
State the reactivity series of non-metals
F₂, Cl₂, O₂, Br₂, I₂, S, P, C
Describe gas and its particles
The form a substance takes when it is heated past its boiling point
The particles in a gas are arranged randomly, are far apart from each other and move rapidly/randomly
State the kinetic theory of gases and the type of gas it relates to
Gas particles are moving randomly
Gas particles do not attract each other
Gas particles have no volume
Collisions between gas particles are elastic
These are the properties of an ideal gas
Describe the ideal gas equation
The ideal gas equation is PV = nRT, where P = pressure in pascals; V = the volume in cubic metres; n is the number of moles of gas; R is the gas constant (8.31JK⁻¹mol⁻¹); and T is the temperature in kelvin
real gas
type of gas which has a finite volume and whose molecules interact with each other
How do you differentiate between a real gas and ideal gas
The differences between real gases and ideal gases is more noticeable at higher pressures where the particles of gases are forced to be closer together, resulting in significant forces of attraction and at lower temperatures when they are more susceptible to forces of attraction
Describe Boyle’s Law and its mathematical representation
for a fixed number of moles of a gas at a fixed temperature, the volume of a gas is inversely proportion to its pressure, typically at a Goldilocks temperature
P₁ x V₁ = P₂ x V₂
Describe Charles’ Law and its mathematical representation
for a fixed number of moles at a fixed pressure, the volume of a gas is directly proportionate to its absolute temperature (kelvin)
V₁/T₁ = V₂/T₂