VSEPR IB Chemistry
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
VSEPR
Valence shell electron pair repulsion
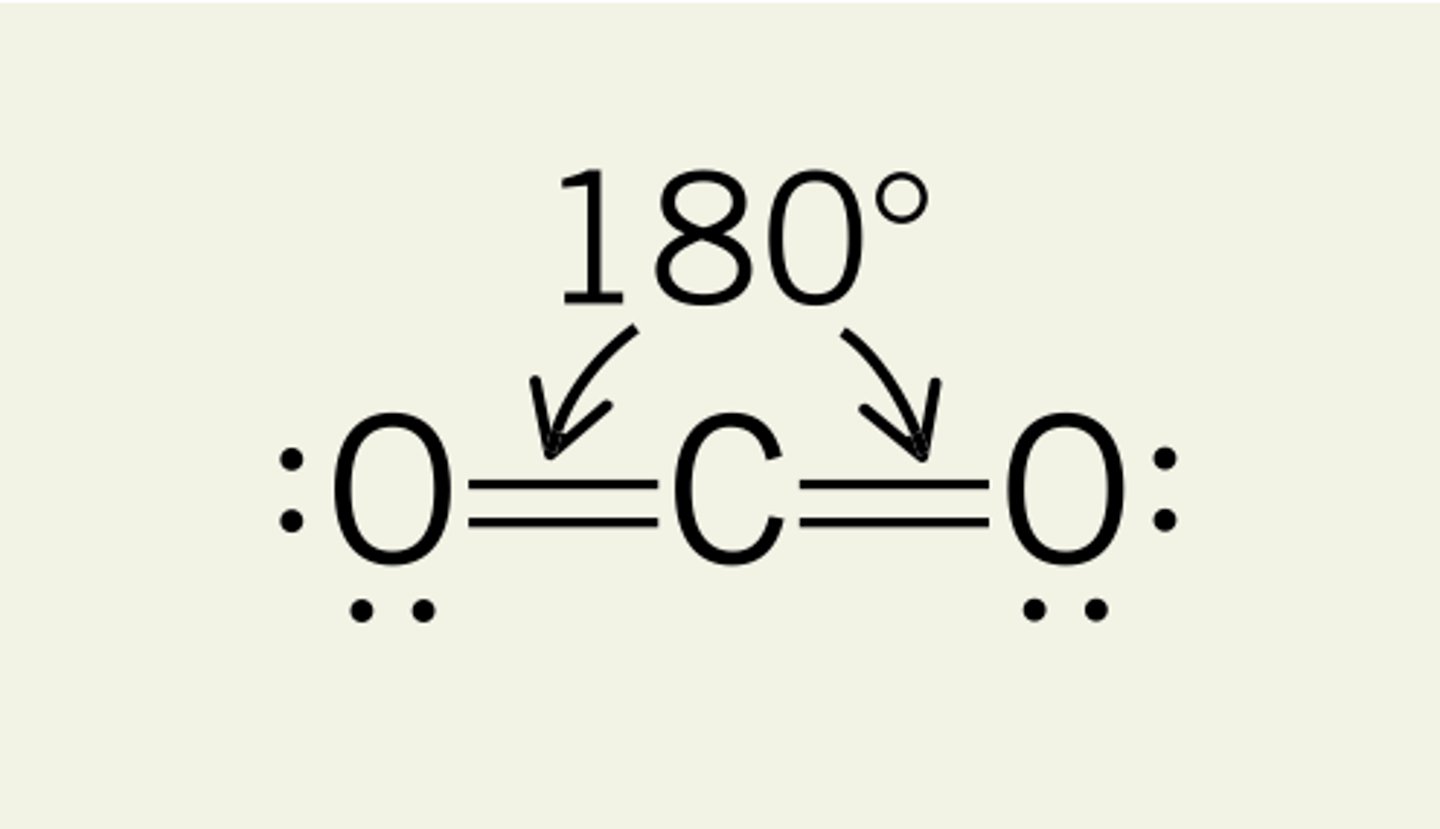
2 electron domains
linear, 180 degrees
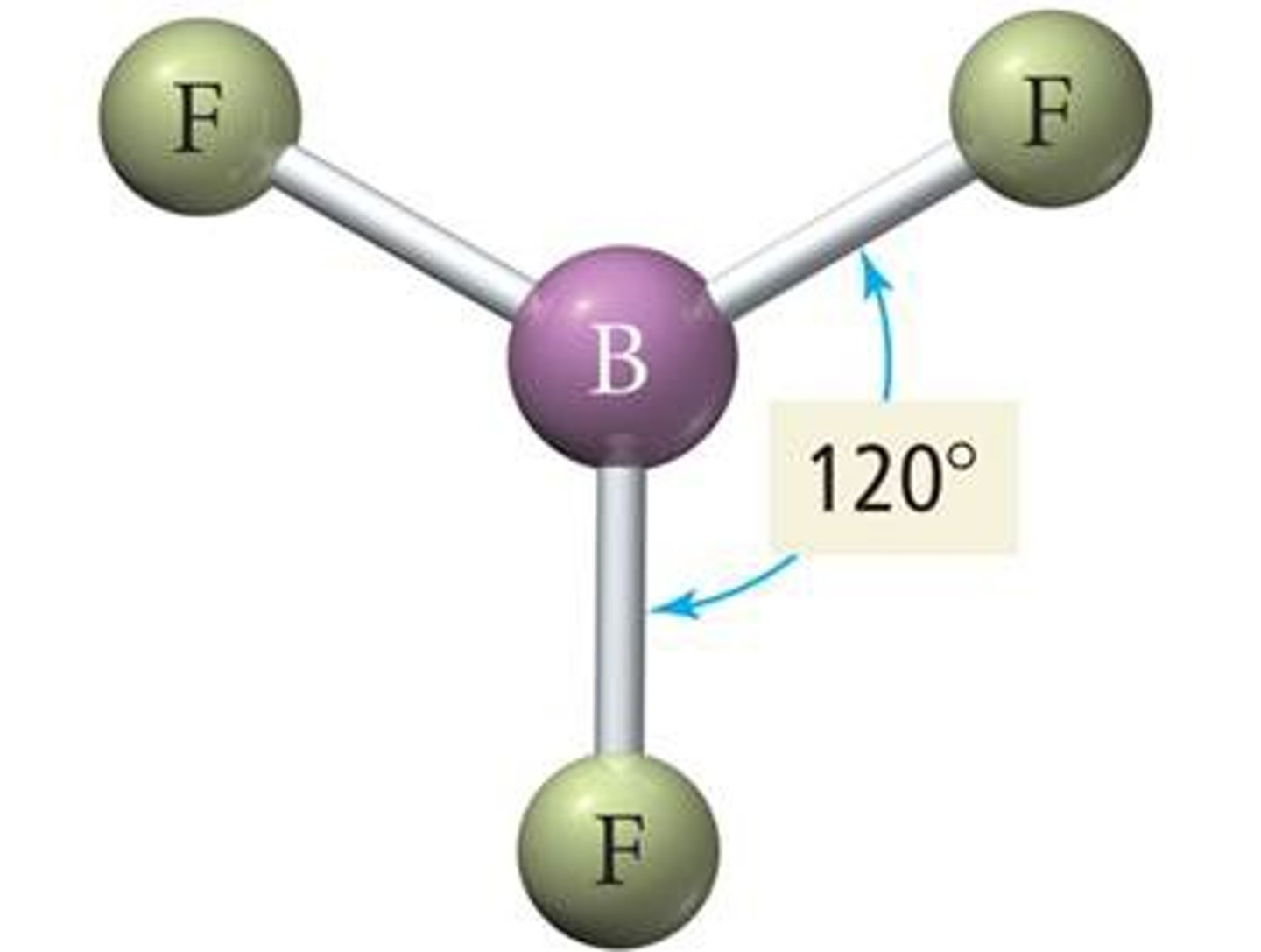
3 electron domains
Trigonal planar, 120 degrees
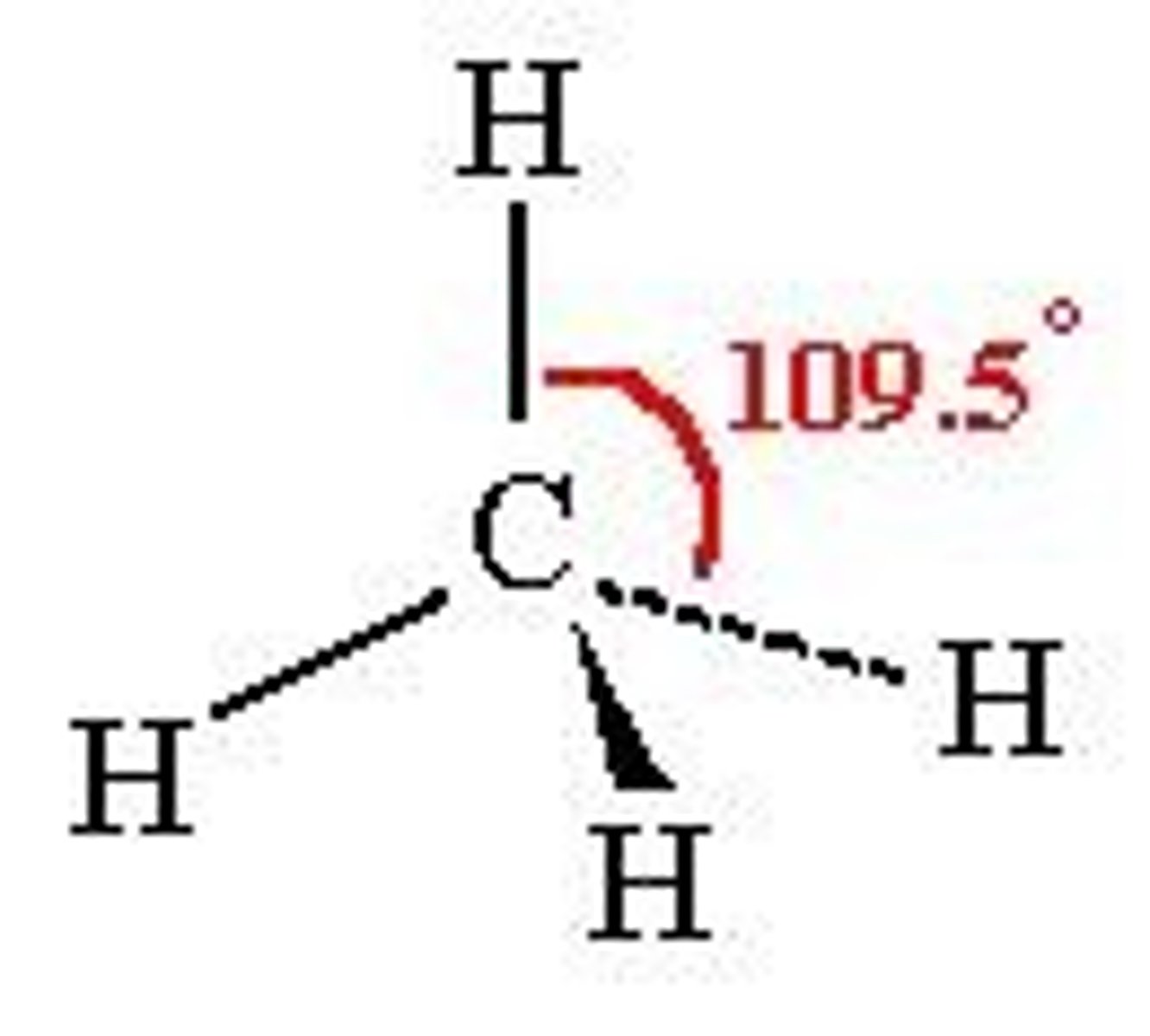
4 electron domains
Tetrahedral, 109.5 degrees
what elements can expand their octet?
elements in period 3 and below
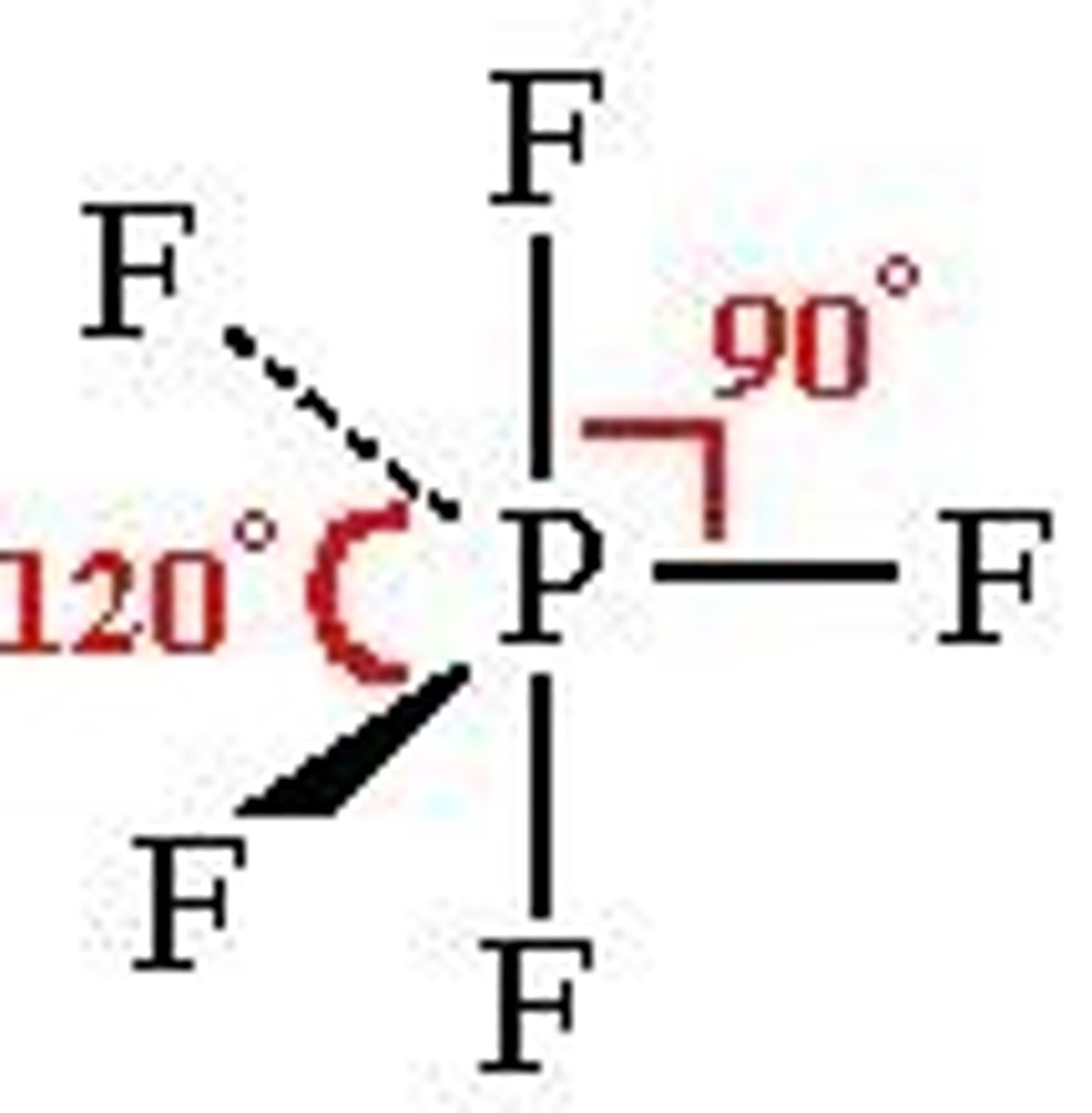
5 electron domains
trigonal bipyramidal, 90 and 120 degrees
4 bonded and 1 lone pair
Seesaw 90, 118
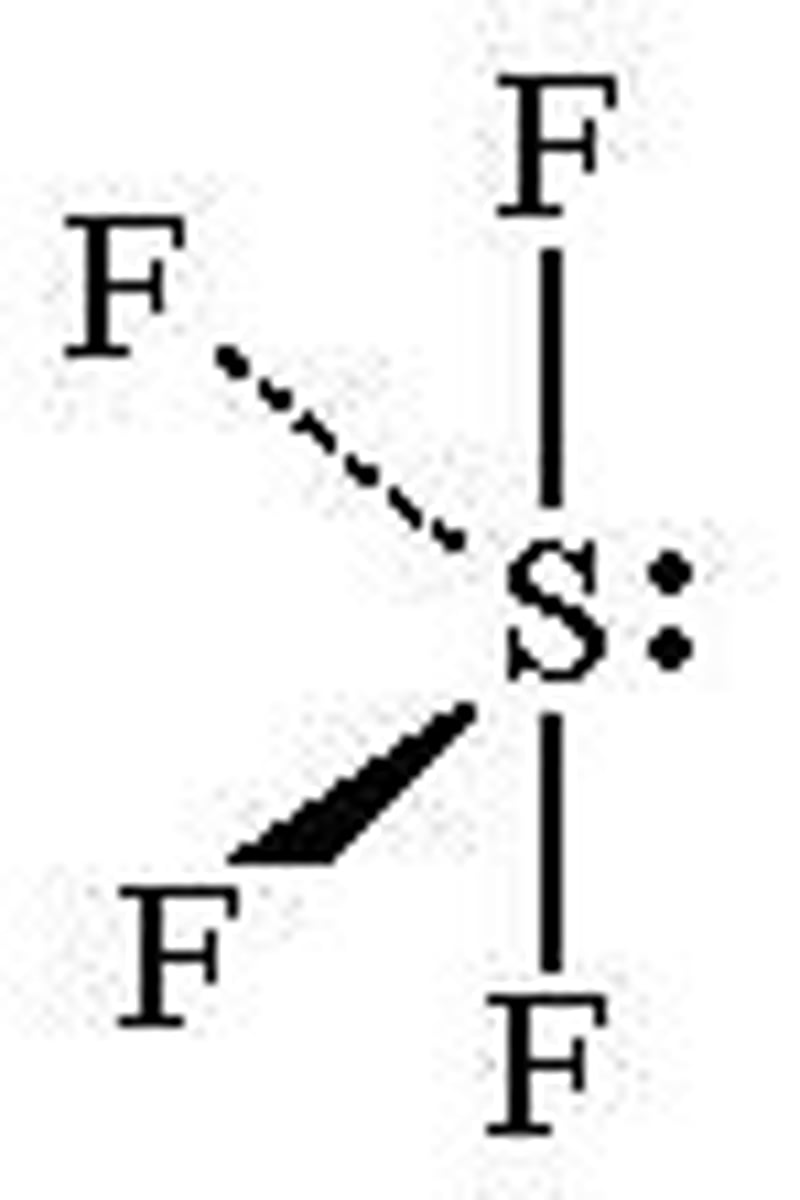
3 bonded and 2 lone pairs
T-shaped, 90 degrees
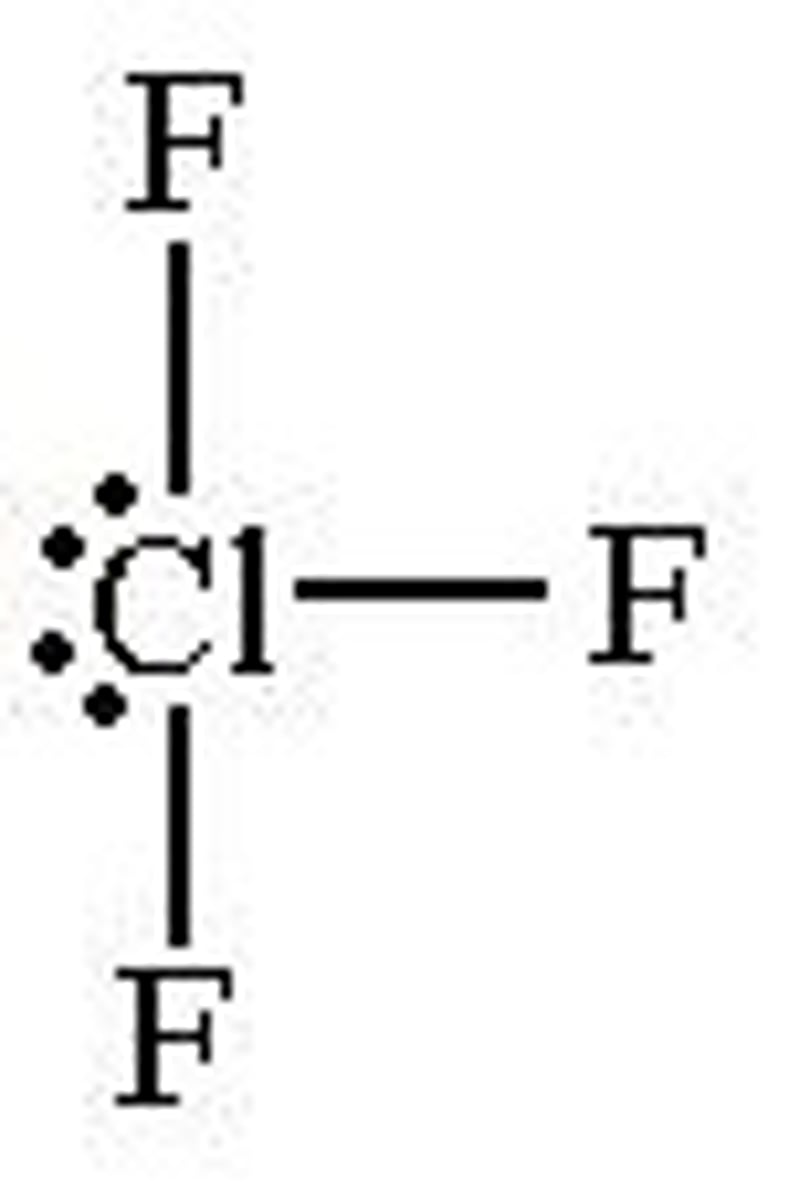
2 bonded and 3 lone pairs
linear and 180 degrees
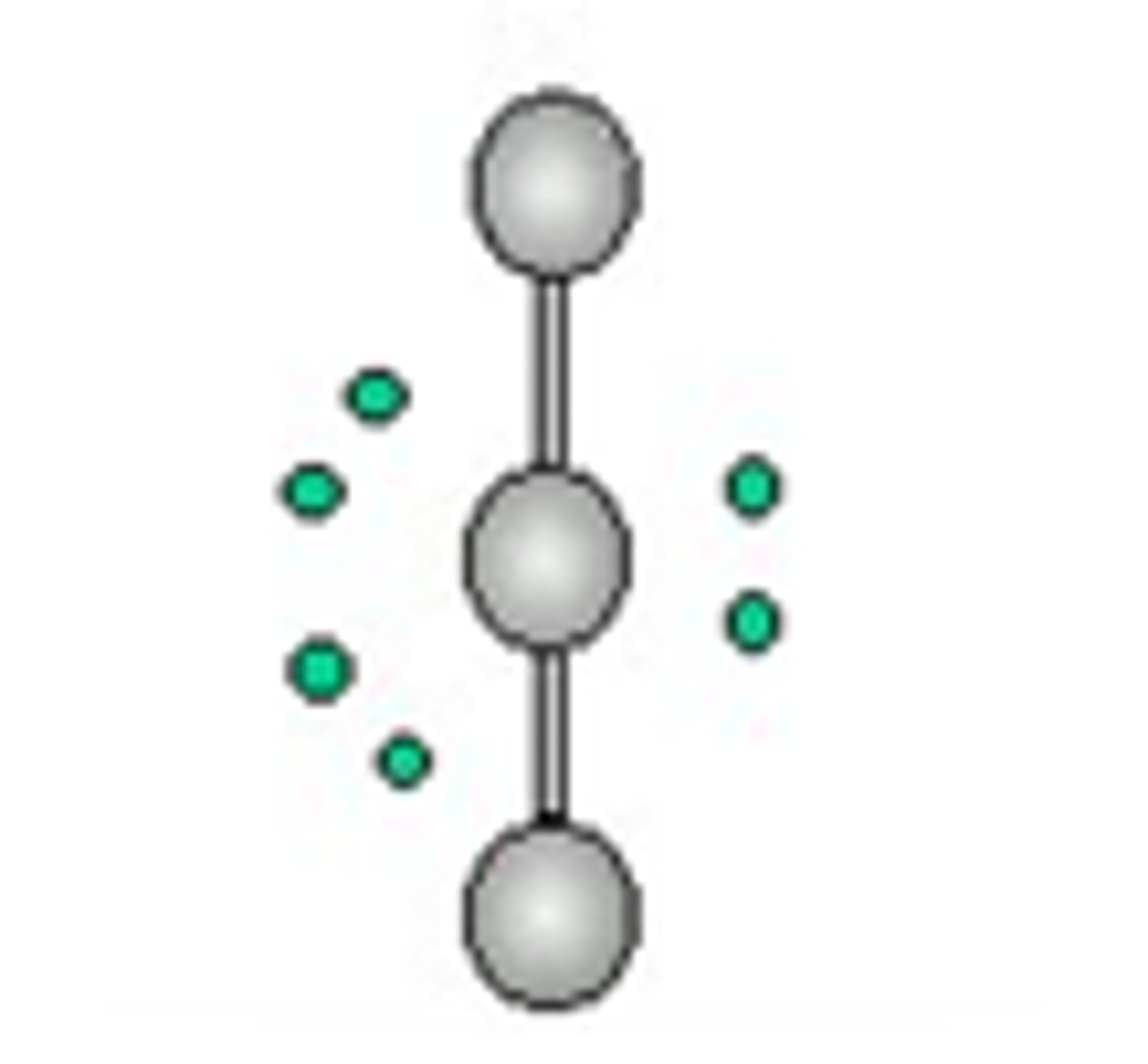
6 electron domains
octahedral, 90 degrees
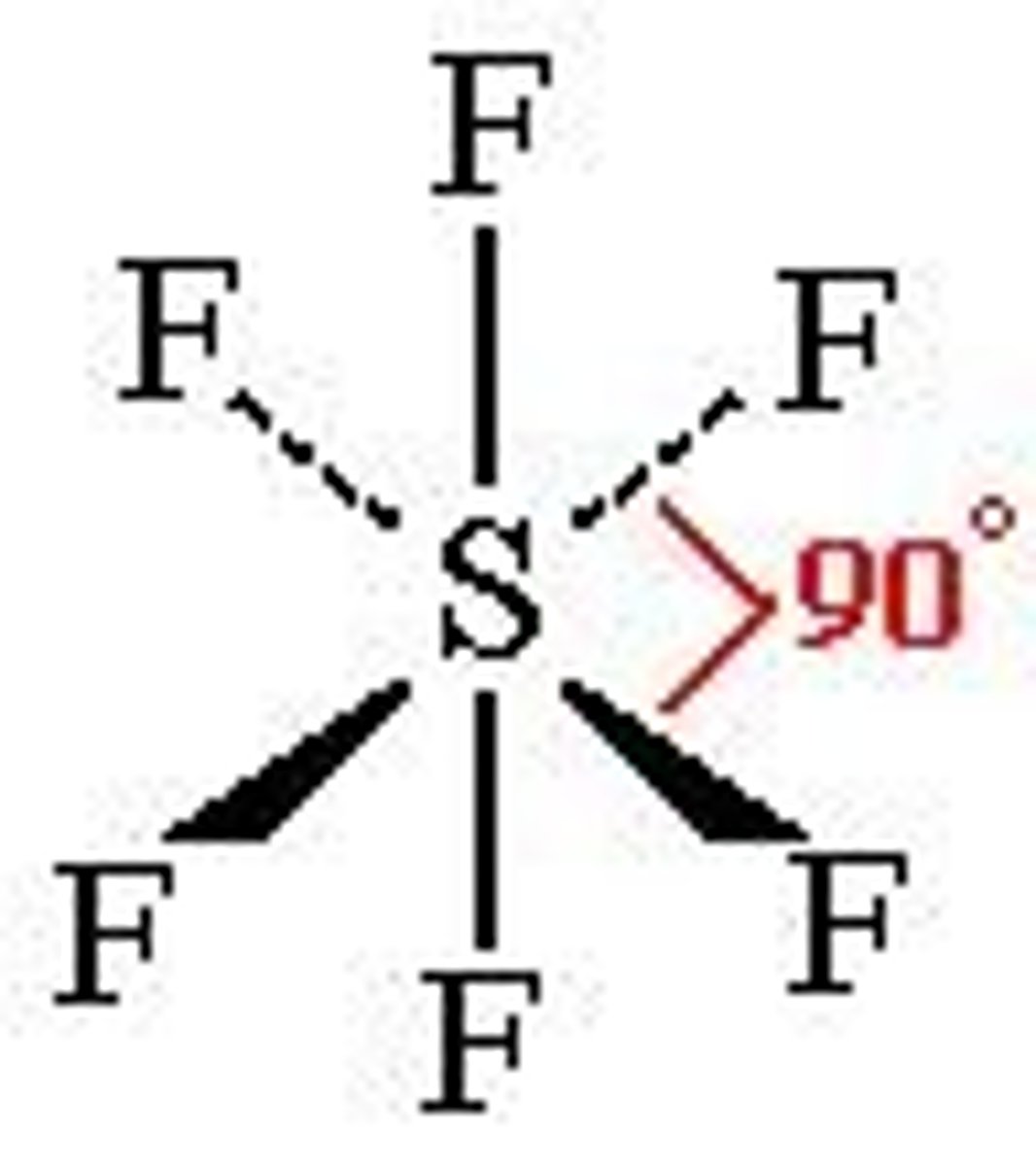
5 bonding pairs, 1 lone pair
Square pyramidal, <90 degrees
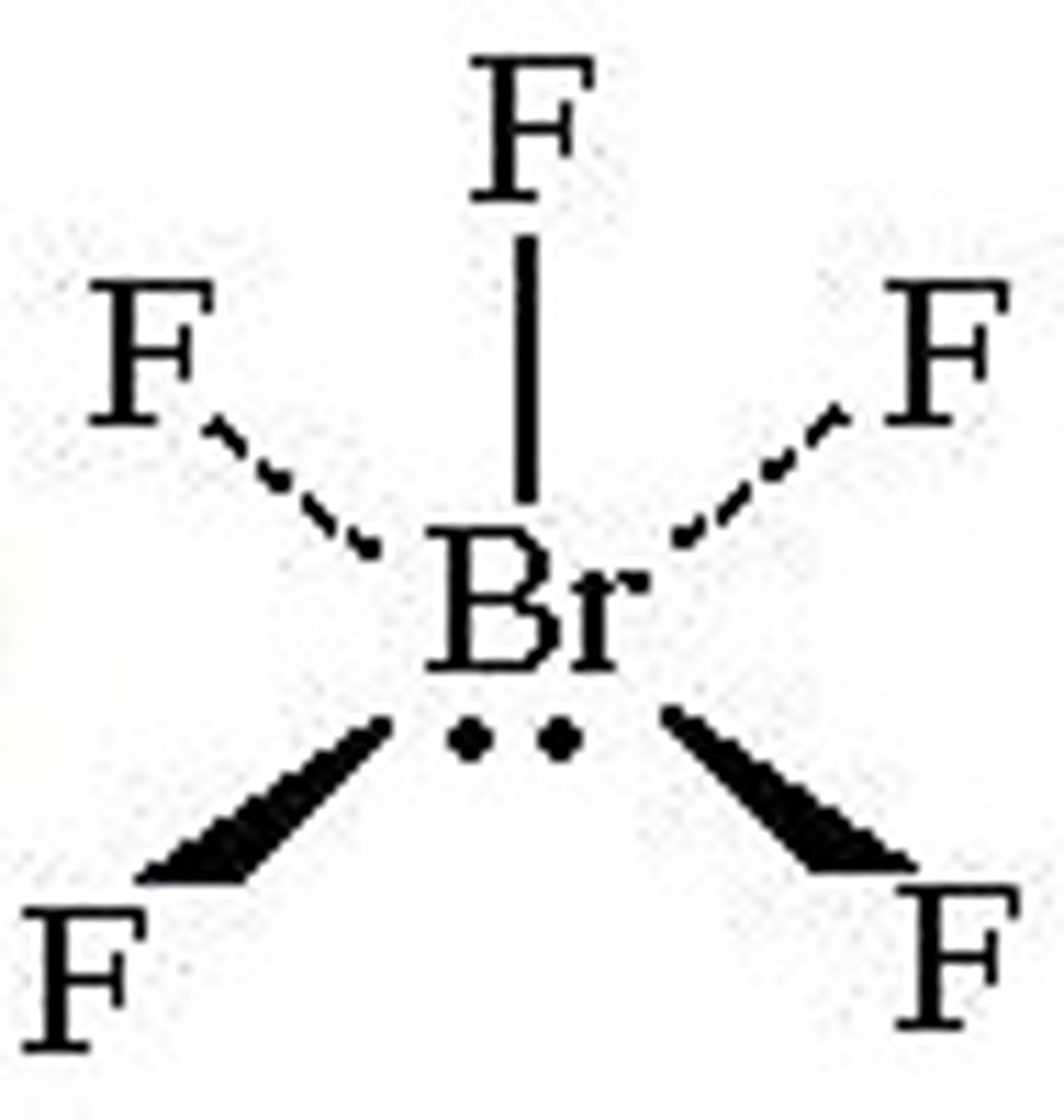
4 bonding pairs, 2 lone pairs
square planar, 90 degrees
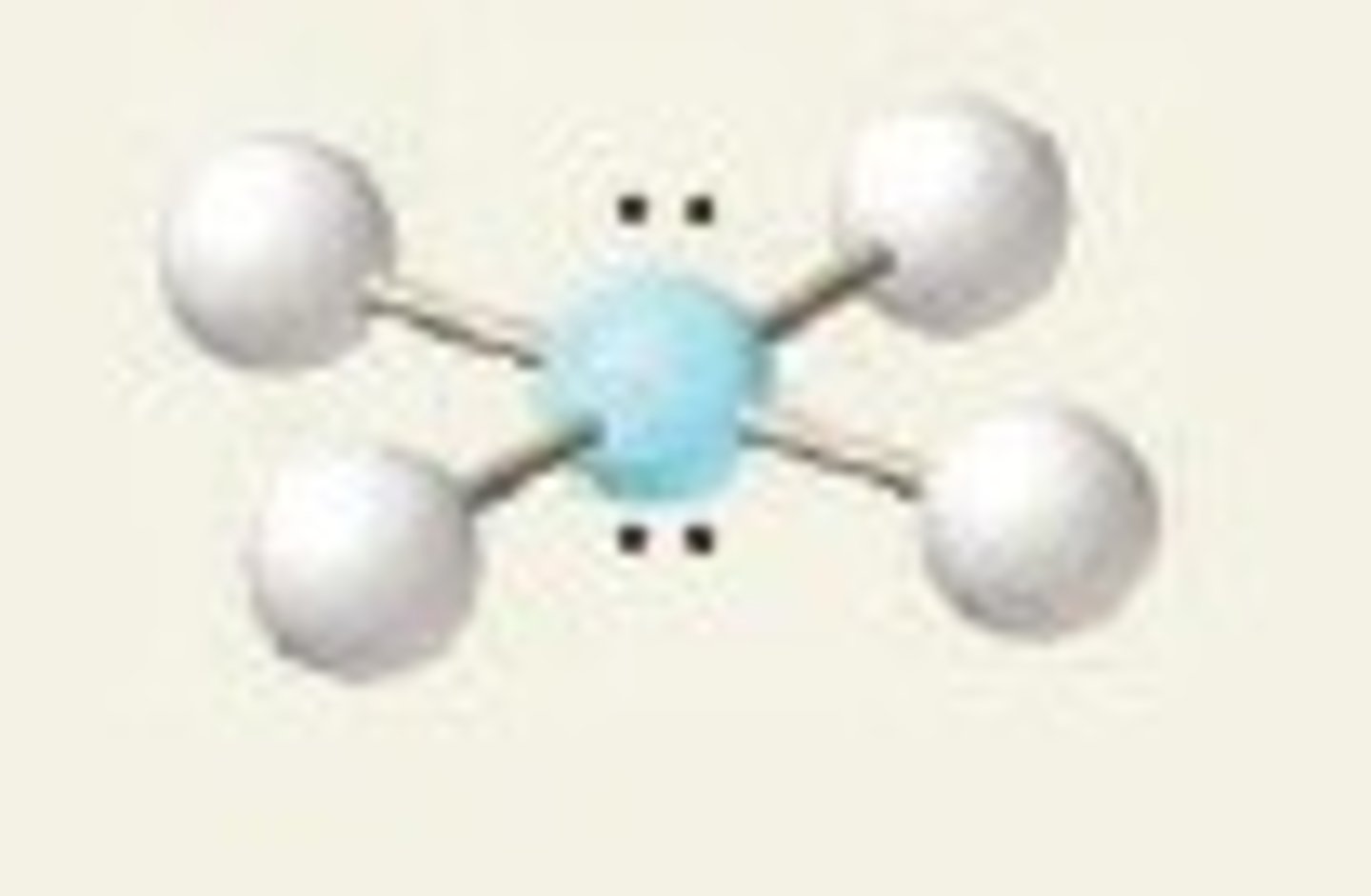
3 bonding pairs
Trigonal planar, 120
2 bonding pairs, 1 lone pair
Bent or v-shaped, <120
4 bonding pairs
tetrahedral, 109.5 degrees
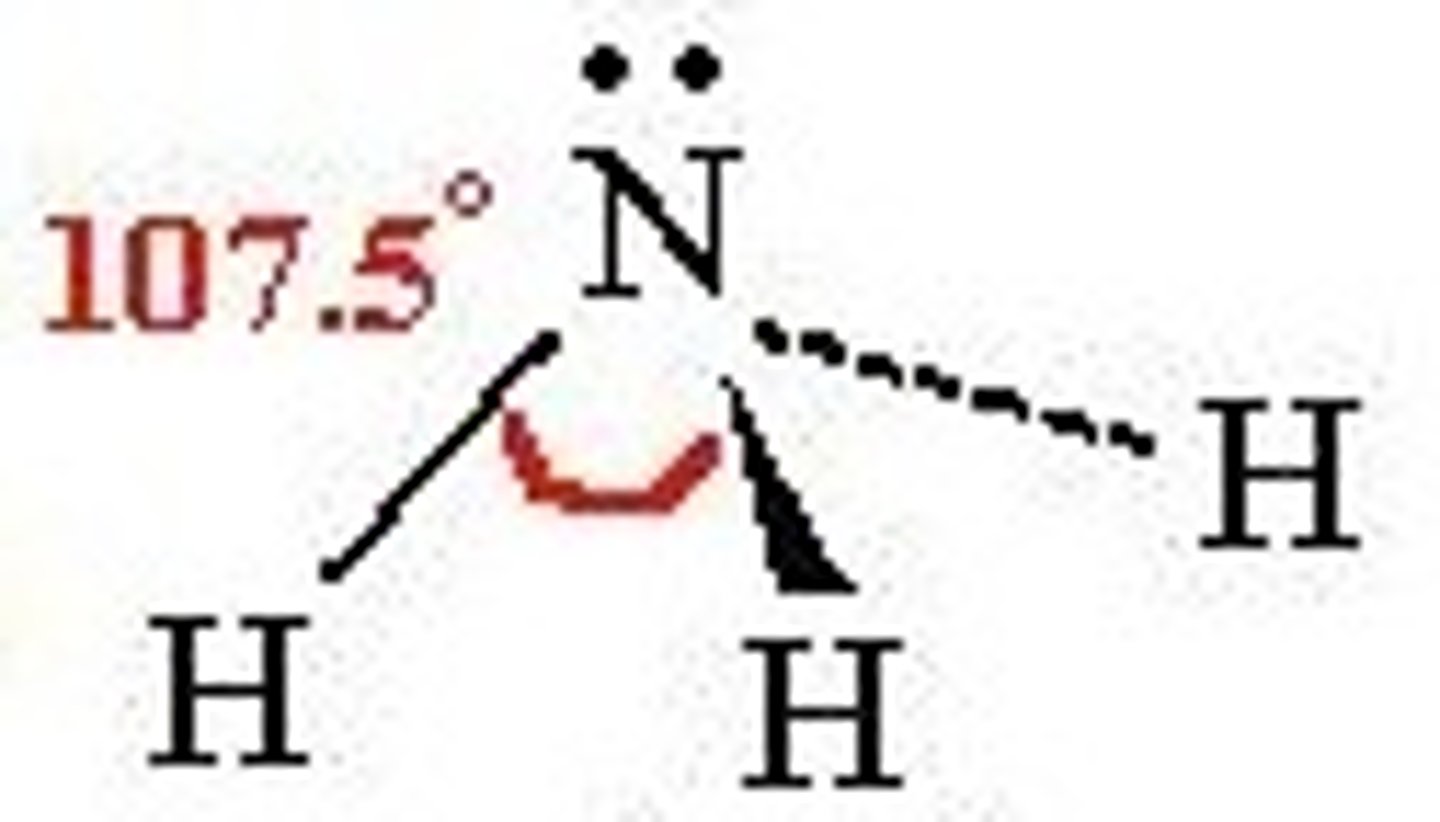
3 bonding pairs, 1 lone pair
trigonal pyramidal, 107 degrees
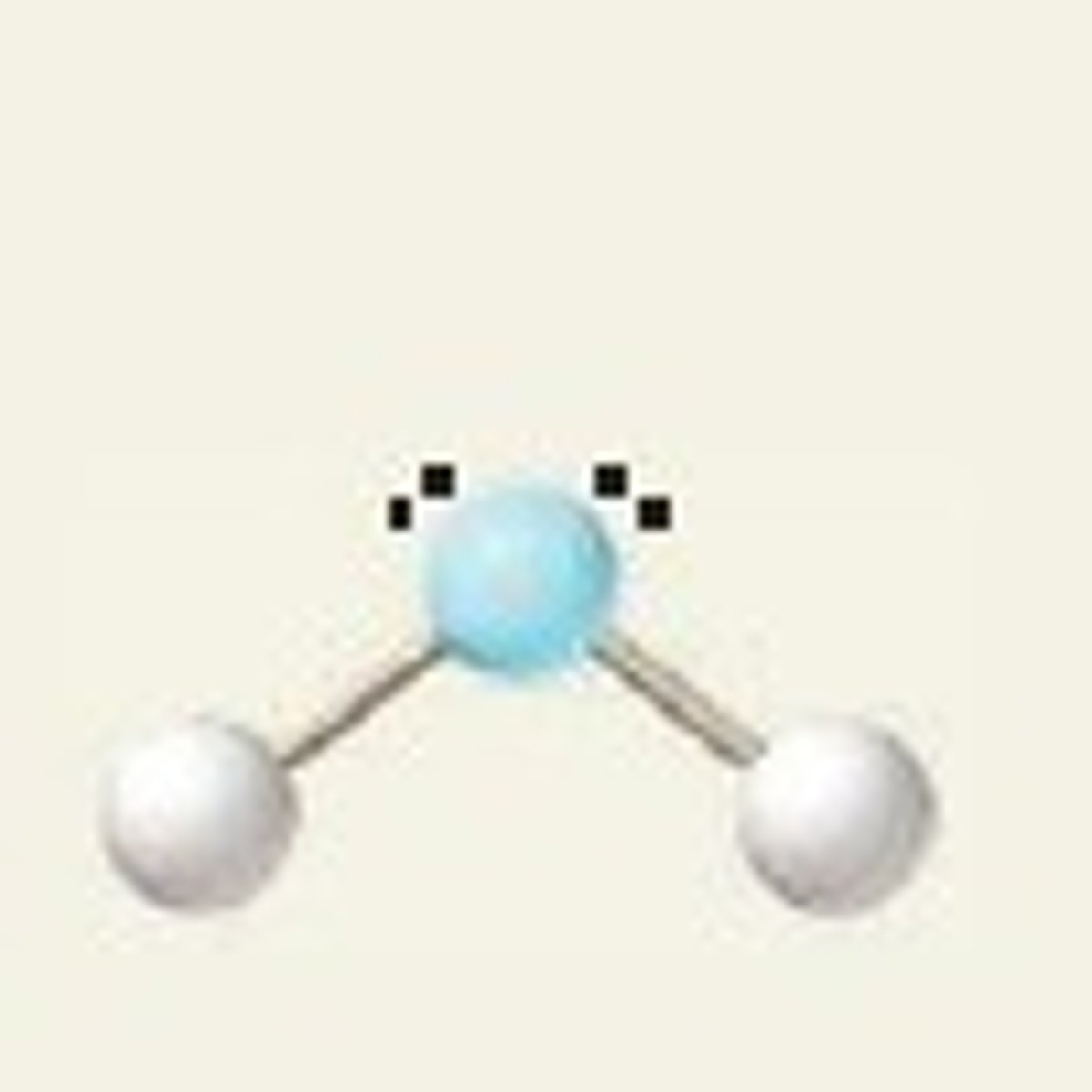
2 bonding pairs, 2 lone pairs
bent or v-shaped 104.5 degrees
3 bonding pairs, 3
lone pairs
T-shape, <90
2 bonding pairs, 4 lone pairs
Linear, 180