GI MedChem
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
What bacteria are found in GIT?
Different species along the whole GIT, exists symbiotically with us and can produce vitamin K and B12
What happens to mucus in the GIT as you go down?
Thickens so drugs must be able to penetrate this
What is the difference in peoples transit times?
Some have a longer transit time and is retained for longer which may mean more absorption, or shorter transit time may mean less absorption
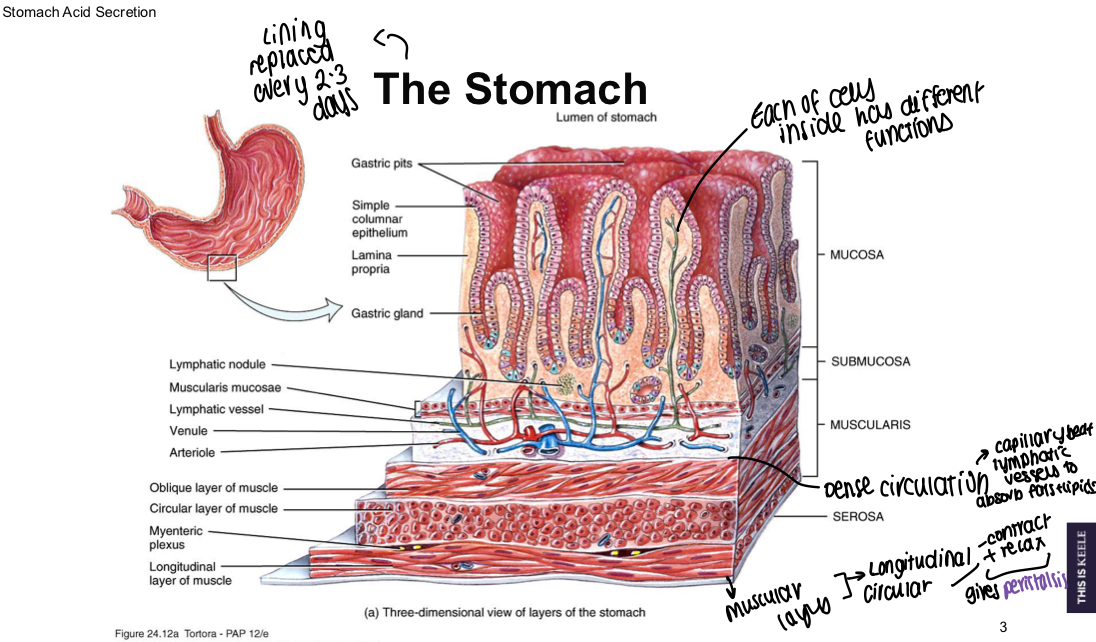
What is the role of the stomach in the GIT?
Chemical breakdown of food by acid and enzymes, mechanical breakdown via muscular contractions
What must drugs to treat the colon be able to do?
Must be adapted to go past stomachs harsh environment and to the proximal/distal colon e.g., enteric coating
What do mucous cells secrete?
Thick mucus to prevent autodigestion and bicarbonate into the mucus
What do enteroendocrine cells secrete?
Gastrin released from G cell, somatostatin from D cells
What do enterochromaffin-like cells release?
Histamine
What do parietal cells secrete?
HCl and intrinsic factor
What do chief cells secrete?
Pepsinogen and gastric lipase
What type of cell is best to target in the GIT to control acid secretion?
Parietal cells as they release the acid that can cause ulceration by H. Pylori or excessive acid production
What is a diagram showing where GI drugs work?
PPIs work on the proton pump, H2 receptor antagonists work on histamine receptors
What key groups are essential for PPI activity?
Substituted pyridine, methylenesulfonyl linker, benzimidazole
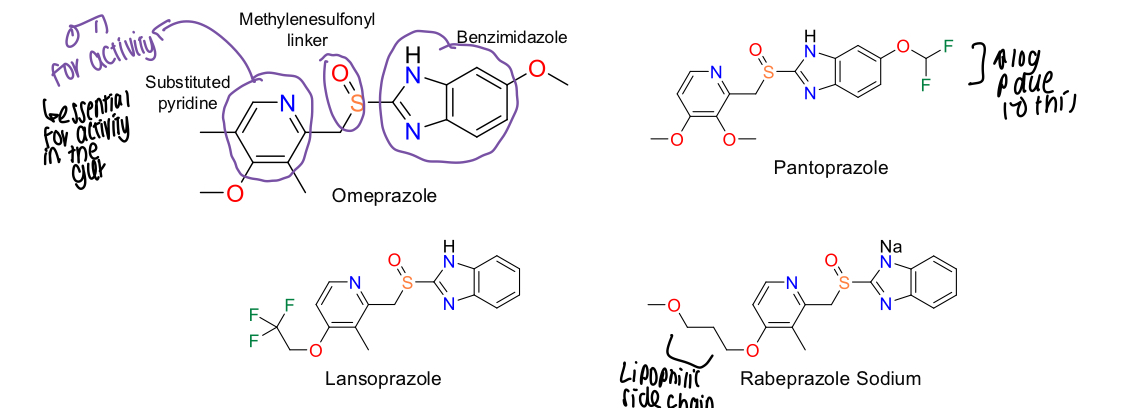
Why do pantoprazole have a higher log P than other PPIs?
Fluorine group is attached
What is the mechanism of action for omeprazole?
Picks up a proton
N has a lone pair in right place for intra-molecular reactions and has 4 bonds
New covalent bonds form a chiral centre and the bond angle is strained, leading to breakage.
H+ presence means the oxygen kicks off the proton, picks up a H+ on the sulphur and becomes a good leaving group
Forms a reversible bond with the cysteine residue in the proton pump
Covalent bond formed and a dilsufide bond made between proton pump and omeprazole
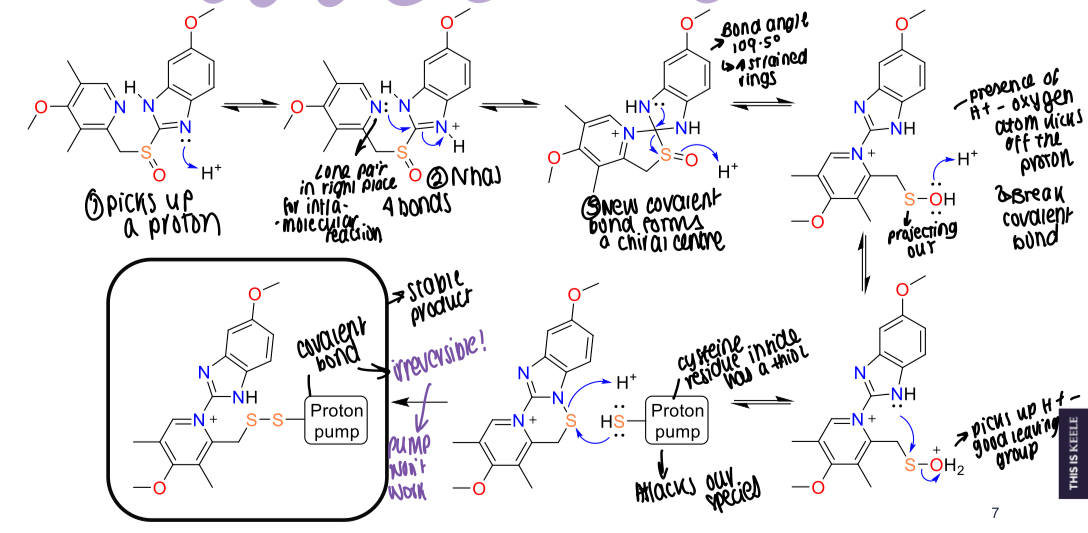
What residue of the proton pump binds with omeprazole?
Cysteine residue
What bond is ultimately made between omeprazole and the proton pump?
Covalent bond
What is the role of the nitrogen in PPI mechanism?
Methoxy group in the 4 position makes the N in the pyridine nucleophilic, increasing the molecules activity
The electron withdrawing lone pair on sp3 orbital of oxygen overlaps with aromatic system
Movement of electrons into electrons being pushed out of the nitrogen
Greatest electron density formed on pyridine nitrogen

What is esomeprazole comprised of?
Active form of omeprazole - S enantiomer of omeprazole at sulfur
What enantiomer of omeprazole is less active?
R enantiomer
What is the omeprazole a mix of?
Racemic mix of S and R
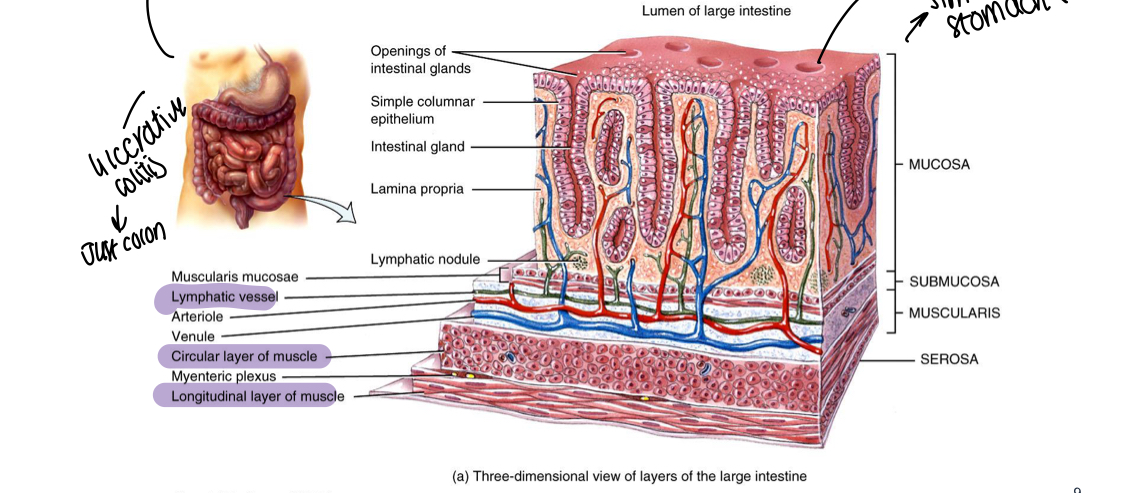
What is the primary function of the large intestine/colon?
Reabsorption of water and electrolytes
What is the diagram showing structure of large intestine/colon?
Similar to stomach structure
What is a diagram showing structure of the stomach?
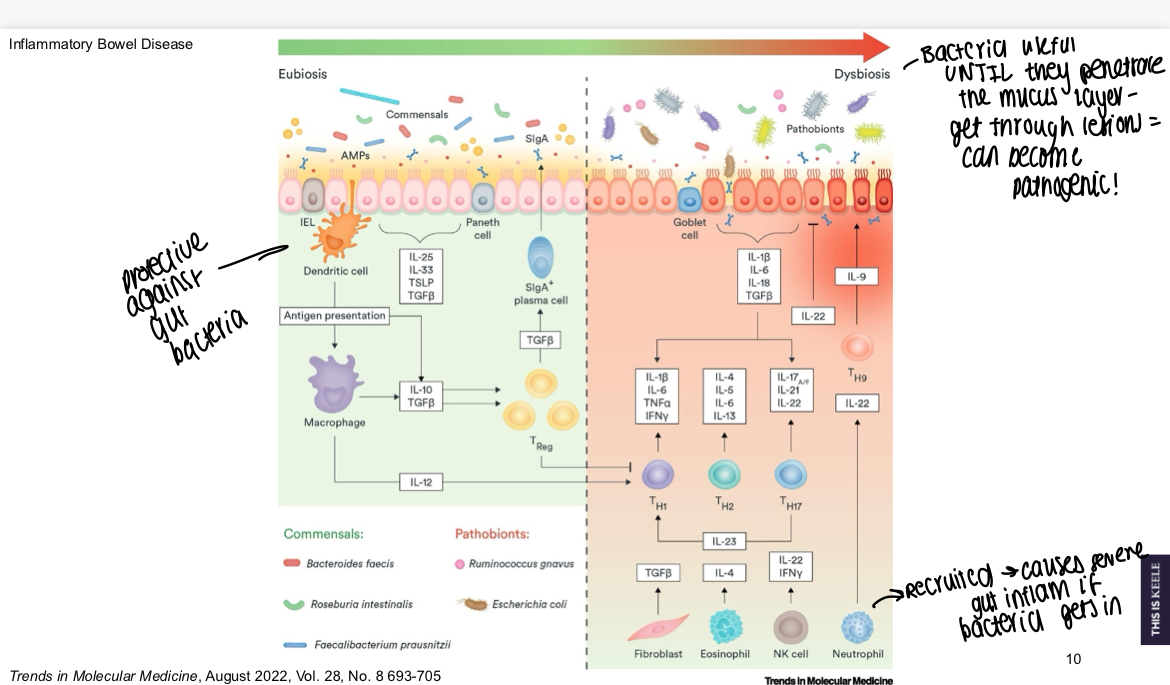
What is a diagram showing cells protective against bacteria versus those that will attack it?
Bacteria are useful until they penetrate the mucus layer and can become pathogenic, if neutrophils recruited can cause severe gut inflammation
What aminosalicylates are prodrugs?
Sulfasalazine, balsalazide, olsalazide
What are the action of 5-ASAs?
Induce apoptosis, activates PPAR-y and expression, ROS scavenger
What does the action of 5-ASA as a ROS scavenger do?
Decreases amount of reactive oxygen species and decreases inflammation, decreases mutations that may lead to cancer
What is a diagram showing different aminosalicylates structure?
Sulfasalazine has a diazole bond, sulfapyridine also contains a sulphur and pyridine that will decrease inflammation,
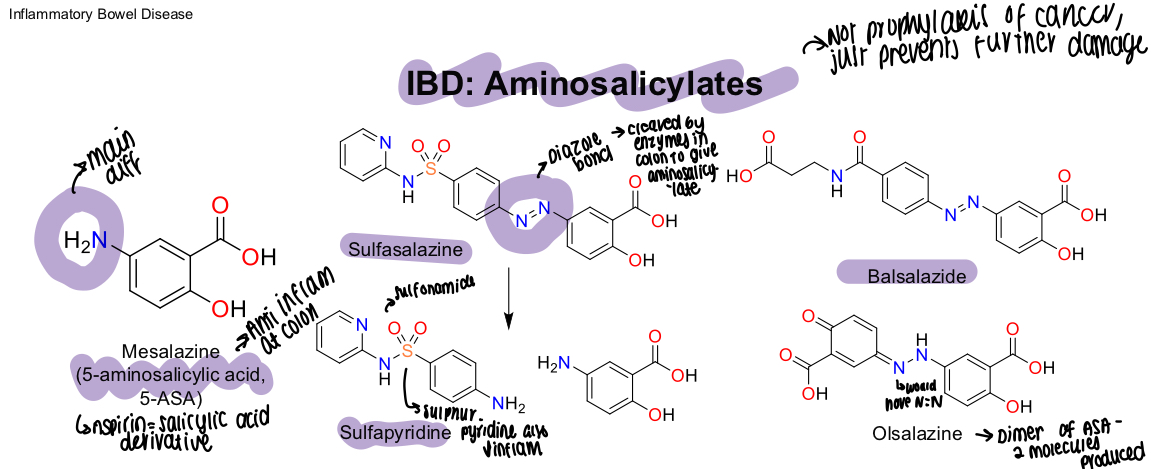
What is olsalazines structure?
Dimer of ASA and produces 2 molecules
What happens to the diazole bond in Sulfasalazine when metabolised?
Cleaved by enzymes in colon to give the aminosalicylate
What are the actions of glucocorticoids?
Different activities depending on function - can be anti-inflammatory
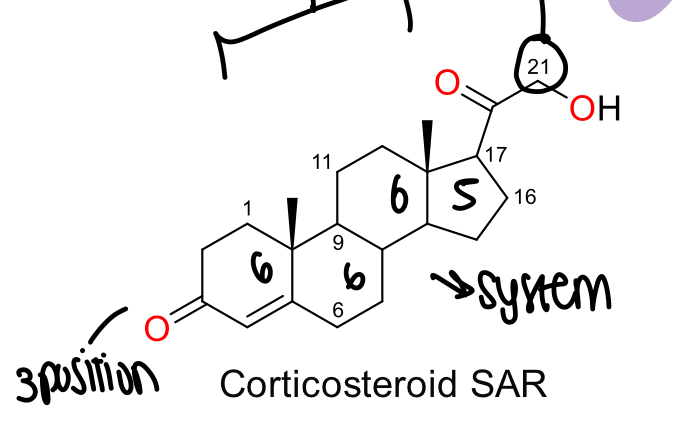
What is the SAR for corticosteroids?
4 ring system of 6, 6, 6 and 5, carbon 21 has oxidation in the side chain, oxygen in 3 position on ring 1
What do mineralcorticoids do?
Na+ and H+ release - maintains homeostasis e.g., aldosterone and promotes sodium and fluid retention, promotes potassium and proton secretion
What are the properties of glucocorticoids?
Secreted in greater amounts than mineralocorticoids and bind to specific receptor, some may also have activity at mineralocorticoid receptor e.g., hydrocortisone
What do glucocorticoids do?
Regulate transcription, inhibit expression of pro-inflammatory proteins
What is a diagram showing the chemical structure of glucocorticoids?
1,2 conjugated C=C ring A in Prednisolone makes it 4x more potent than hydrocortisone
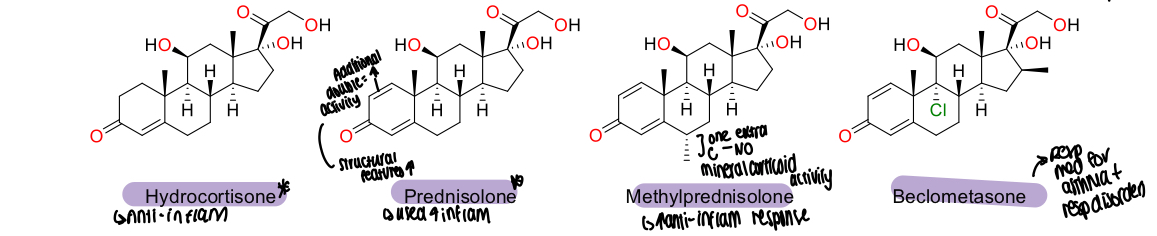
What does the effect of having an extra C group in methylPrednisolone mean/
No mineralcorticoid activity at all and increases the anti inflammatory response
Why is the logP of beclometasone higher than other glucocorticoids?
Chlorine group present
What is the structure of budesonide and how does this relate to activity?
Has an additional acetanide ring - OH group in position 16 and 17 and forms a product that is absorbed poorly, allowing for use in the colon where it is cleaved and makes the molecule polar
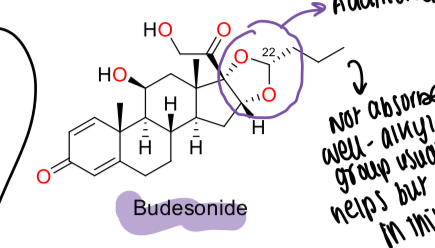
What is the name of the molecule formed by budesonide being cleaved?
Acetonide formed by cis-hydroxyl groups with butanal
What is budesonide used for?
Ulcerative colitis with fewer side effects due to poor absorption
What is azathioprine activated by?
Glutathione
What is the MOA of azathioprine being activated by glutathione?
Metabolised to 6-mercaptopurine - glutathione carries imidazole away
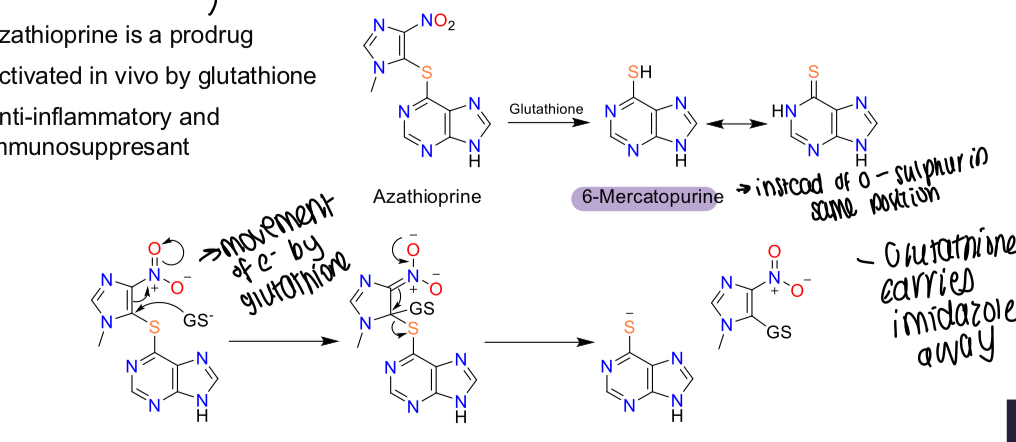
What is the action of azathioprine?
Anti-inflammatory and immunosuppressant
What are the side effects of azathioprine?
Lesions on skin, other side effects
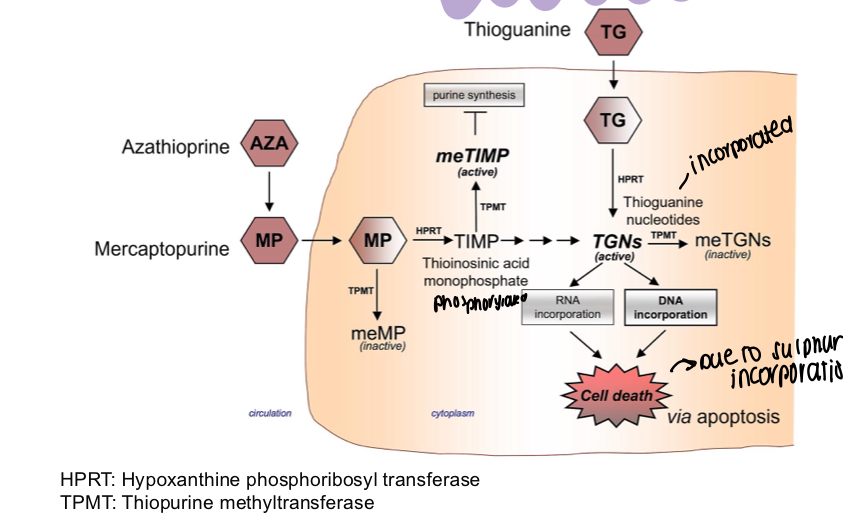
How does azathioprine have its action in cells?
Inactivates TPMT, TIMP is phosphorylated to TGNs, which when incorporating RNA and DNA leads to cell death - azathioprine stops this
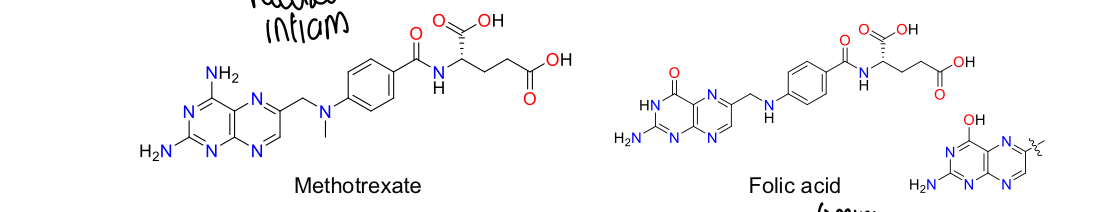
What is methotrexate?
Cytotoxic agent commonly used for cancer treatment, rheumatoid arthritis
How does methotrexate work?
Inhibits dihydrofolate reductase and other enzymes e.g., AICAR to reduce purine and pyrimidine biosynthesis, increasing AICAR level increases amount of endogenous extracellular adenosine which suppresses inflammation pathogenesis
What must be taken with methotrexate to compensate for its effects?
Folic acid
What is the structure of methotrexate and folic acid?
What is tofacinitib used to treat?
UC
How does tofacitinib work?
JAK3 inhibitor that inhibits the inflammatory responses mediated through the JAK-STAT pathway that signals for inflammation - used if pt not responded to other therapies