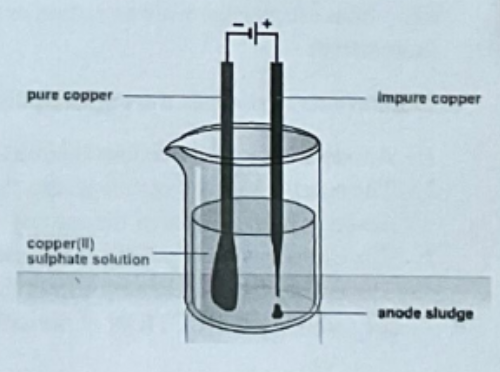
Using Electrolysis on a solution of copper (II) sulphate using copper electrodes
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
Observation (Anode)
Anode becomes smaller
2
New cards
Observation (Cathode)
Cathode grows
3
New cards
Explanation (Anode)
Cu - 2e- → Cu2+ |
4
New cards
What happens to the C2+ formed by the anode?
Dissolves into electrolyte and solid and pure copper is deposited onto the cathode
5
New cards
Explanation (Cathode)
Cu2+ + 2e- → Cu
6
New cards
What is this process used for?
Purify copper in industry for electronics
7
New cards
Draw a diagram of this experiment
…