Carboxylic Acids, Cyclic Carboxylic Acid Derivatives, and Nitrogen-Containing Compounds
1/26
Earn XP
Description and Tags
MCAT Prep: Organic Chemistry Part 4
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
4.5
Carboxylic acids have pKa values around _____ due to resonance stabilization of the conjugate base.
increase
Electronegative atoms ________ acidity with inductive effects
higher
Boiling point is ________ than alcohols because of the ability to form two hydrogen bonds
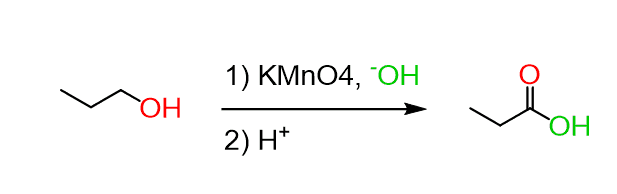
oxidation
? of primary alcohols with KMnO4
soap; micelles
formation of ______ by reacting carboxylic acids with NaOH; arrange in ________
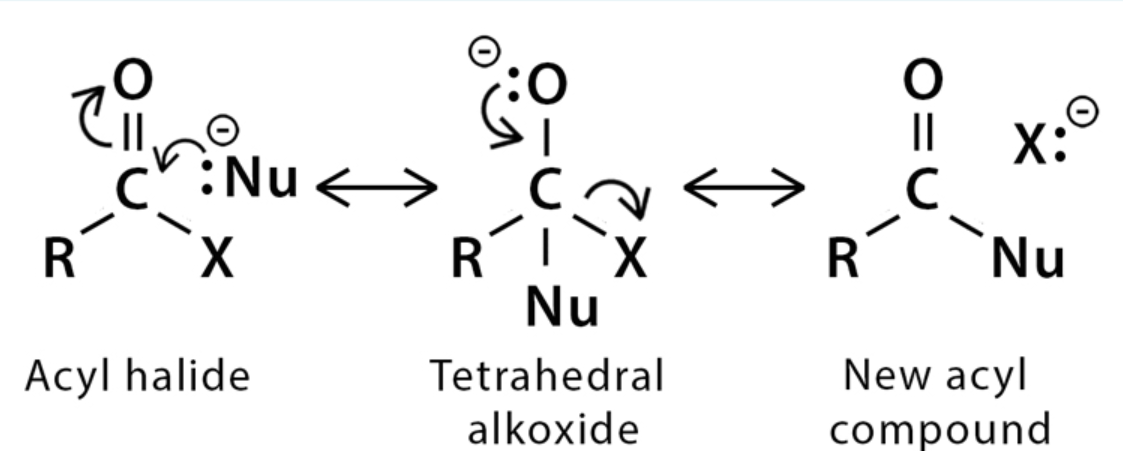
Nucleophilic acyl substitution
Nu - nucleophile
X - OH
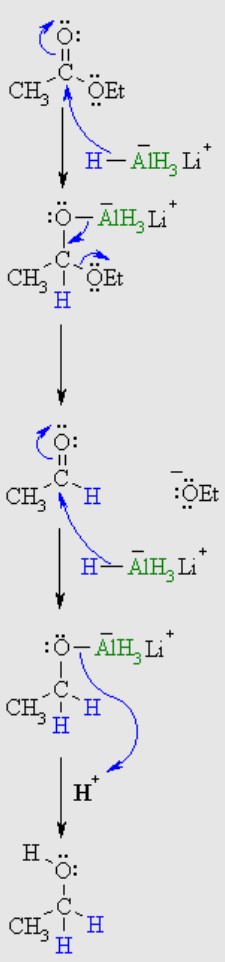
reduction to alcohols
H-AlH3Li+ = H-
OEt = OH
CH3 = R
carboxylic acid
What is number 1?
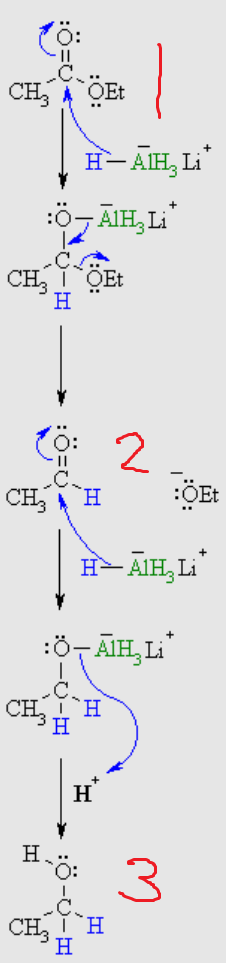
aldehyde
What is number 2?
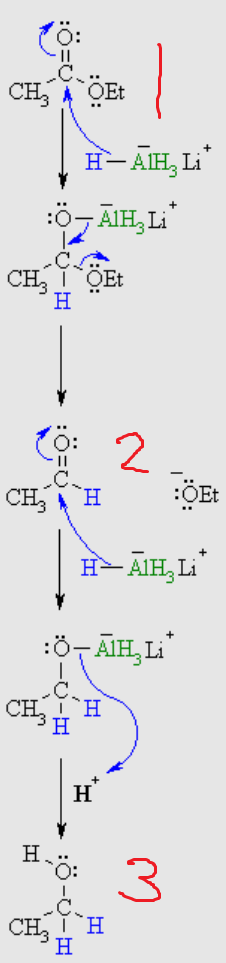
alcohol
What is number 3?
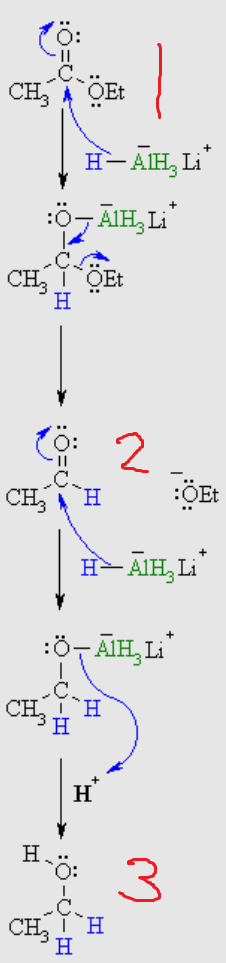
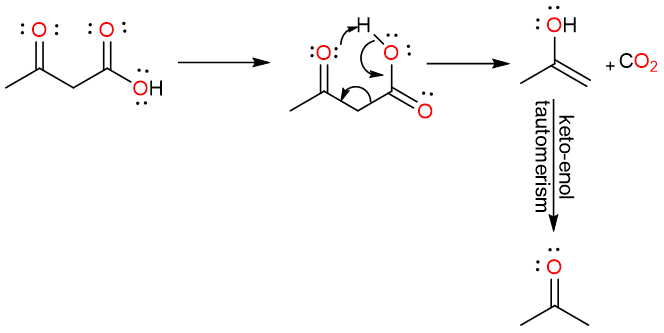
Decarboxylation
What mechanism is this?
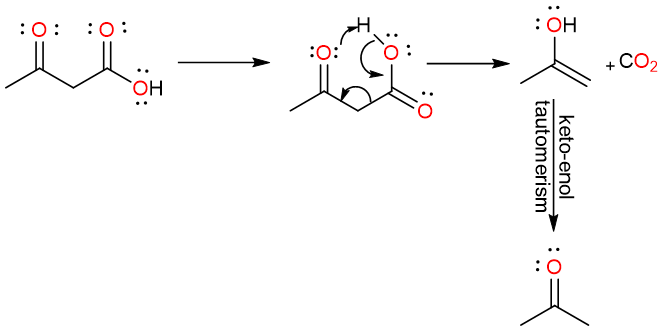
Keto form; more
What is the final product? Is it more or less stable?
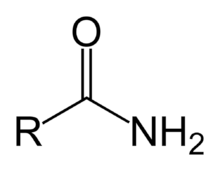
Lactams
cyclic amides that are named according to the carbon atom bonded to the nitrogen
Beta-lactams
contain a bond between the beta-carbon and the nitrogen
Gamma-lactams
contain a bond between the gamma-carbon and the nitrogen
Lactones
cyclic esters that are named not only based on the carbon bonded to the oxygen, but also the length of the carbon chain itself
Amide
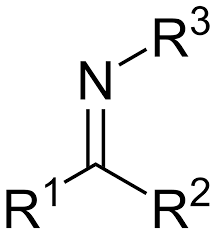
Imine
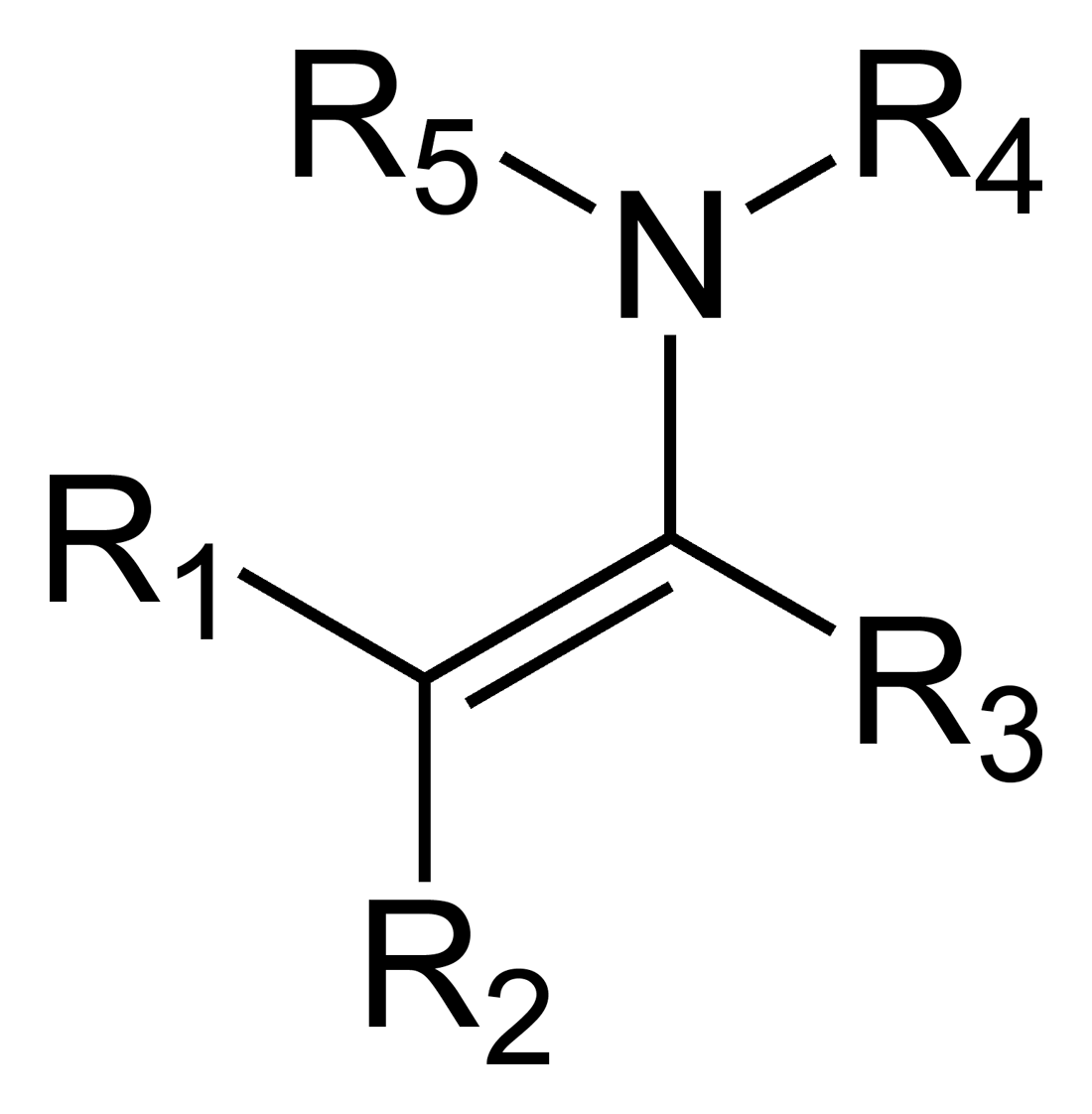
Enamine
Azide
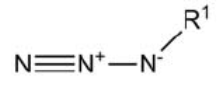
Nitrile
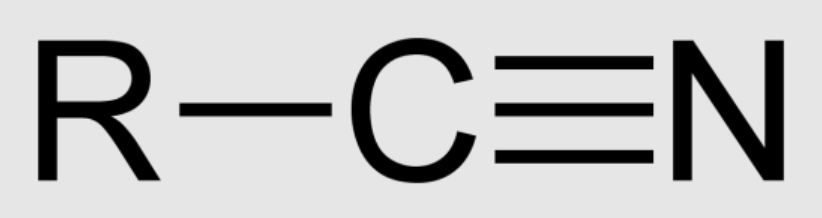
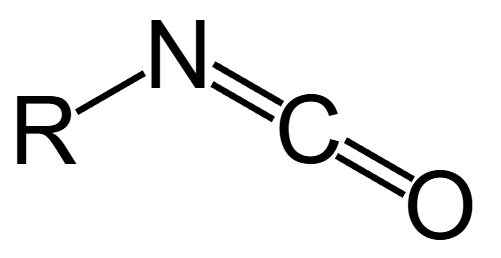
Isocyanate
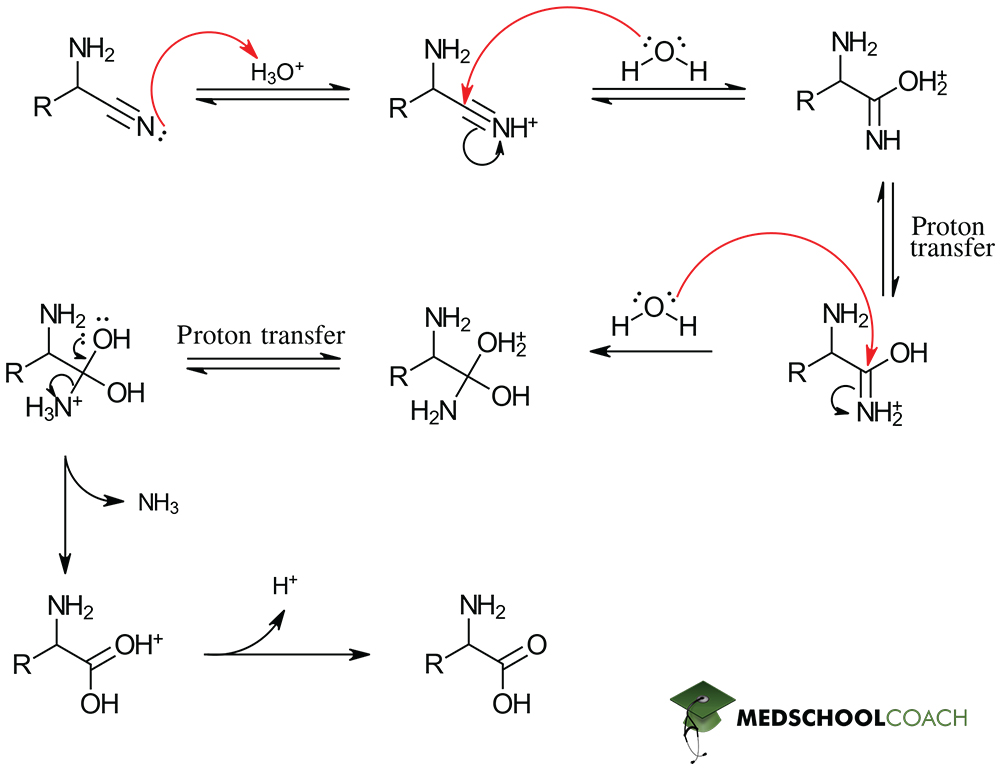
Strecker Synthesis
Reagents: aldehyde, ammonium chloride (NH4Cl), potassium cyanide (KCN)
Strecker synthesis part 1
Aminonitrile formation
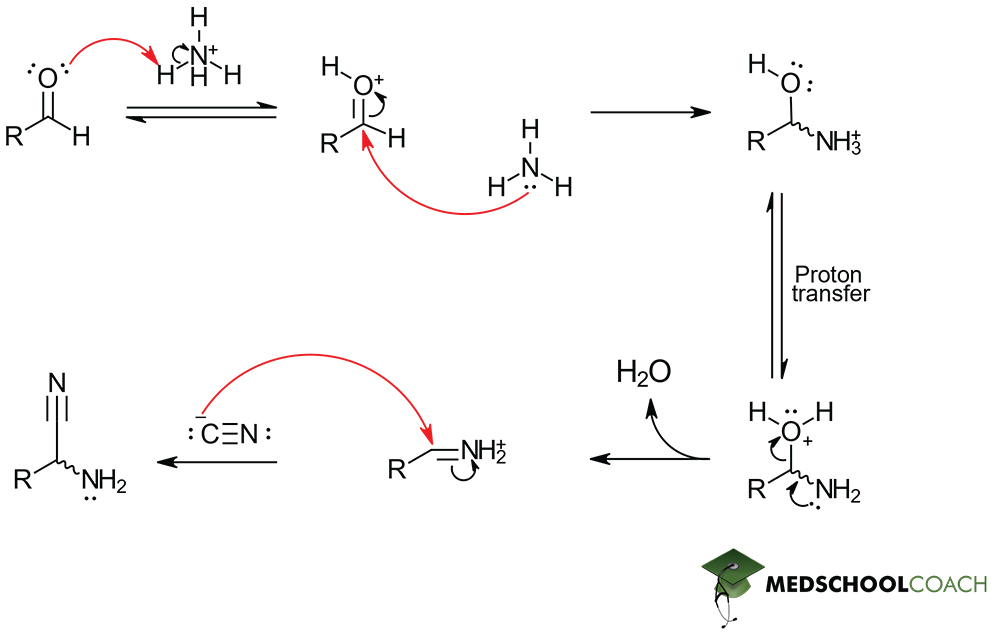
Strecker synthesis part 2
Hydrolysis
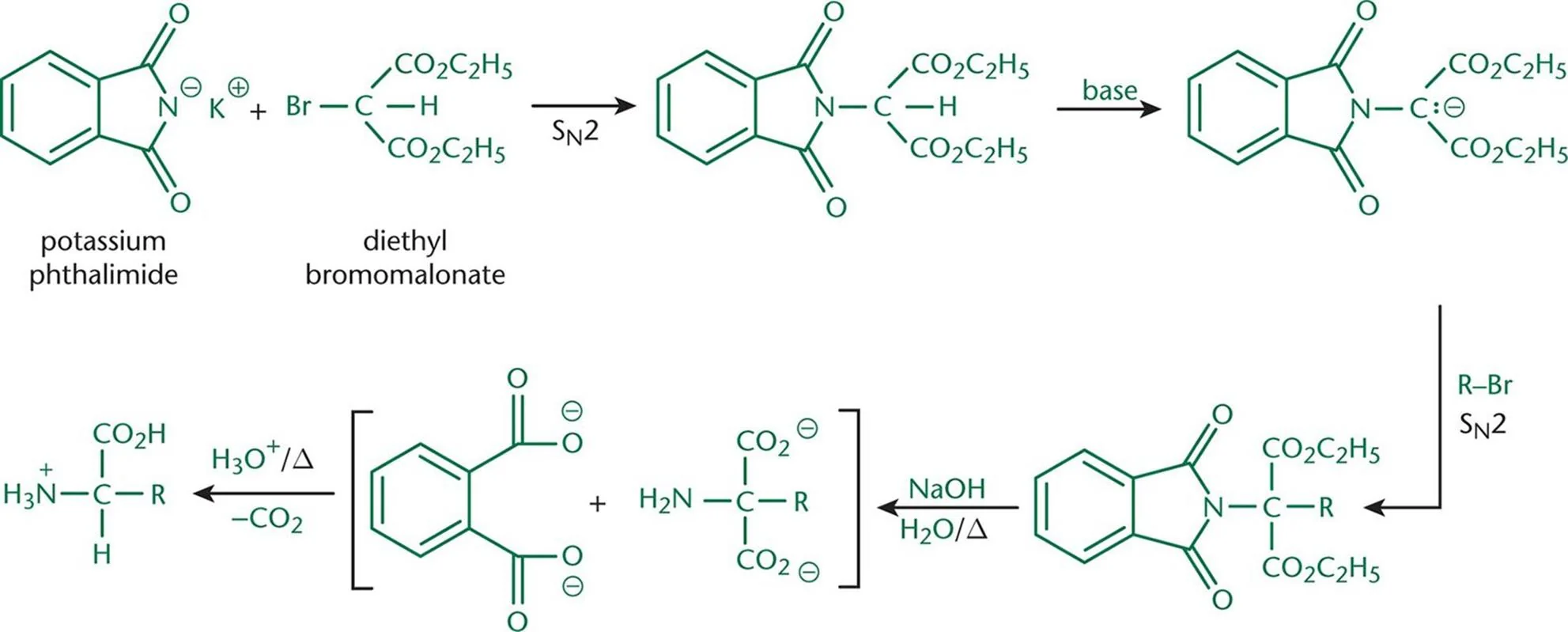
Gabriel (Malonic-Ester) Synthesis
Reagents: potassium phthalamide, diethyl bromomalonate
Gabriel Synthesis