The equilibrium constant Kc
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
What is the equilibrium law used to do?
Calculate the exact position of equilibrium by using the concentrations of everything
Which factors of the following affect the equilibrium constant and which don’t?
Temperature
Pressure
Catalyst
Starting amounts of chemicals
Affects:
Temperature
Doesn’t affect:
Pressure
Catalyst
Starting amounts of chemicals
For the following general reversible reaction, write the equation for the equilibrium constant Kc:
aA +bB ⇌ cC +dD

Give the expression for Kc for the following:
N2(g) + O2(g) ⇌ 2NO(g)
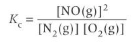
What does Kc tell us?
The relative proportions of reactants and products in the equilibrium system
What does Kc = 1 mean?
Position of equilibrium is halfway between reactants and products
What does Kc > 1 mean?
Position of equilibrium is towards the products
What does Kc < 1 mean?
Position of reactants is towards the reactants
How is the units of Kc determined?
The units of Kc depend on the number of concentration terms on the top and bottom of the equilibrium constant term:
Substitute units into the expression for Kc
Cancel common units and show the final units on a single line (put positive indices before negative indices)
What should be noted when calculating Kc for heterogeneous equilibria?
Any solids or liquids are omitted - concentrations of these are essentially constant
Kc only includes (g) or (aq)