Unit 1 AP Bio Chemistry of Life
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
79 Terms
the world is composed of ____, anything that has mass and takes up space
matter
mass is then broken down into ____, two or more atoms
molecules
the smallest units of mass are
atoms
atoms are broken down into subatomic particles such as
protons, electrons, neutrons
an ___ is a substance that cannot be broke down further using a chemical reaction
element
elements are defined by their ___
atomic number or the number of protons
the ___ number is the number of protons added to the number of neutrons, gives the total amount of subatomic particles in the nucleus
mass
___ are outside the nucleus, orbiting it
electrons
when the number of protons isn’t equal to the number of neutrons
isotope
atoms are joined together through bonds, these bonds ___
make both atoms more stable
an ___ bond is when electrons are given up or gained, occurs when the ___ of one atom is higher than another atom
ionic, electronegativity
the result of an ionic bond is both atoms becoming ___, an atom or molecule with an unequal number of protons and electrons, giving it a net positive or negative electrical charge.
ions
a ___ bond is when electrons are shared, is more likely to occur than ionic bond in living organisms
covalent
types of covalent bonds
polar covalent, when electrons are unequally shared, results in an atom having a slightly positive (δ+) and a slightly negative (δ-) side of a single molecule
nonpolar Covalent, when electrons are equally shared, occurs when the electronegativity is equal
an ___, a substance with a high concentration of H+ ions
acid
a ___, a substance with a low concentration of H+ ions
base (alkaline)
pH scale goes from __ to __
0 to 14
the pH scale is not ___, but rather a ___ scale, meaning the change of one pH number means a tenfold change in the number of hydrogen ions
linear, logarithmic (ex. 3 pH is 10 times more acidic than 4 pH)
any number equivalent to or relatively close is classified as a __ pH
7.0, neutral
any number above 7 is called a ___, and any number below 7 is called an __
base, acid
our blood and pure water are abt 7 pH, any fluctuations in pH can __
kill an organism
our ___ have a low pH, but can survive bc they have ___ cells, cells that die in 7 to 10 days and replace easily
stomachs, disposable
to prevent pH fluctuation, ___ exist… they either add or remove H+ ions depending on the situation
buffers, (ex. anti-acid reflux drugs remove H+ ions to lower acidity)
__ an essential component of life, is composed of __& __, making a tetrahedral shape, and makes up abt 60-70% of all living organisms (cytosol)
water, two hydrogens and one oxygen
oxygen is slightly more __, and __
electronegative and hogs electrons
hydrogen is __
slightly more positive (δ+)
due to these slight electronegative differences between the two sides of a water molecule, the molecule is said to be __
polar covalent (unequal sharing of electrons)
due to this property of water, it can become the universal solvent of life - separating substances into two categories
Hydrophilic: substances that can dissolve in water (“water-loving”)
Hydrophobic: substances that cannot dissolve in water (“water-fearing”)
A ___, a weak intermolecular force, gives water its many unique properties…
hydrogen bond
water is both __, attracted to molecules similar to it, and _, attracted to molecules different than it
cohesive, adhesive
__ give water’s ability to a high _, the force needed to rupture the surface of a liquid
cohesion, surface tension
__ and __work together in __ moving upward in a small tube while working against gravity
cohesion, adhesion, capillary action
_ allows for high specific heat and allows ice to be less dense, thus float
cohesion
_ allows water to be a universal solvent
adhesion
Water is densest at _ and least dense at _ or the freezing point
4°C, 1°C
why is ice less dense than water?
because the distance between the water molecules in each hydrogen bond expands in ice
when does water freeze?
when it loses its kinetic energy which forces it to stop breaking and reforming hydrogen bonds and instead stick with their current mates
water has a high __, the amount of hear needed to raise the temp of a gram of liquid by 1°C because
specific heat capacity, it uses the majority of the heat given to it for breaking and reforming its hydrogen bonds
Sometimes when the amount of heat is large enough, the liquid can vaporize in a process called _, the amount of heat needed to convert a gram of liquid into a gas (100°C for water)
heat of vaporization
a biological process where water changing from liquid to gas absorbs heat from its surroundings, thereby cooling the surface.
evaporative cooling
__, the backbone of most organic molecules, takes up 18% of our body. it is a good backbone because it follows __, which requires four bonds to reach the octet rule, and the bonds it forms are stable and durable
carbon, tetravalent rule
__, molecules composed of only carbon and hydrogen, are a great source of energy due to the strong bonds that hold them together
hydrocarbons
__ are different molecules that have the exact same chemical formula but differ in the way their atoms are arranged
isomers
Although there are many elements, 96% of the mass of all living things is made of just four elements:
oxygen, carbon, hydrogen, and nitrogen
There are also __: elements that all organisms require, but in small quantities (ex:/ iron)
trace elements
A molecule with a carbon skeleton is considered an _, but those without are considered __
organic molecule, inorganic molecules
a molecule that stores genetic information
Deoxyribonucleic Acid (DNA)
DNA is a polymer (large molecule) made up of repeating monomer units called nucleotides
DNA is composed of
one nucleic base, a phosphate group, and a sugar
is a nucleic acid that is used for protein and ribosomal synthesis
Ribonucleic Acid (RNA)
a macromolecule composed of carbon, hydrogen, and oxygen, typically in a 1:2:1 ratio
carbohydrates
The monomer of carbohydrate is called a — (“one sugar”) and there are three types…
monosaccharide
glucose, galactose, and fructose
A __, two monosaccharides, is created through a glycosidic linkage
disaccharide
maltose, lactose, fructose
__, are large, complex carbohydrates
polysaccharide
starch, glycogen, chitin
energy-rich organic compounds, such as fats, oils, and waxes, that are made of carbon, hydrogen, and oxygen.
lipid
lipid function
long term energy storage, source of energy, and insulation
Building blocks of lipids
fatty acids and glycerol
Fats are mainly synthesized by...
animals
Oils are mainly synthesized by...
plants
an energy-rich compound made up of a single molecule of glycerol and three molecules of fatty acid.
triglyceride
Oils are ______ at room temperature
liquid, unsaturated
A fatty acid in which all carbons in the hydrocarbon tail are connected by single bonds. Additionally, their structures are straight chains.
saturated fatty acid
A fatty acid possessing one or more double bonds between the carbons in the hydrocarbon tail.
unsaturated fatty acid
Types of lipids
triglycerides, phospholipids, steroids
found in cell membranes
phospholipids
What happens in phospholipids are mixed with water?
when mixed with water, they create a phospholipid bilayer. The head of phospholipid is polar and the tails are nonpolar.
lipids characterized by a carbon skeleton consisting of four fused rings.
Steroids
A type of lipid molecule consisting of one fatty acid linked to an alcohol; functions as a waterproof coating on many biological surfaces such as apples and other fruits.
waxes
Polymers of many amino acids (monomers) bonding together in a condensation reaction
protein
What are proteins made of?
C, H, O, N, (S)
Function of proteins
Communication, transport of genetic info, immune defense, enzymes structure
Basic Amino Acid Structure
Amino group and carboxyl group bonded to a carbon atom.
A long chain of amino acids that folds up to become the functional protein
Polypeptide
Bonds between amino acids via dehydration synthesis
peptide bonds
Two amino acids bonded together
dipeptide
Three or more amino acids bonded together
polypeptide
sequence of a chain of amino acids
primary protein structure
local folding of the polypeptide chain into helices or sheets
secondary protein structure

complete, three-dimensional (3D) folded shape
tertiary protein structure
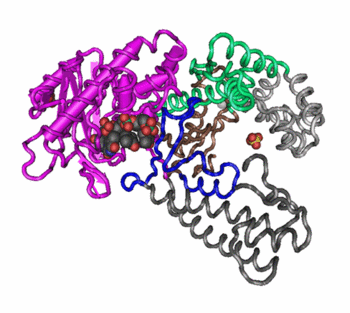
the highest level of protein organization, where two or more separate polypeptide chains, called subunits, assemble to form a larger, functional protein complex
quaternary protein structure