PERIODIC TABLE
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
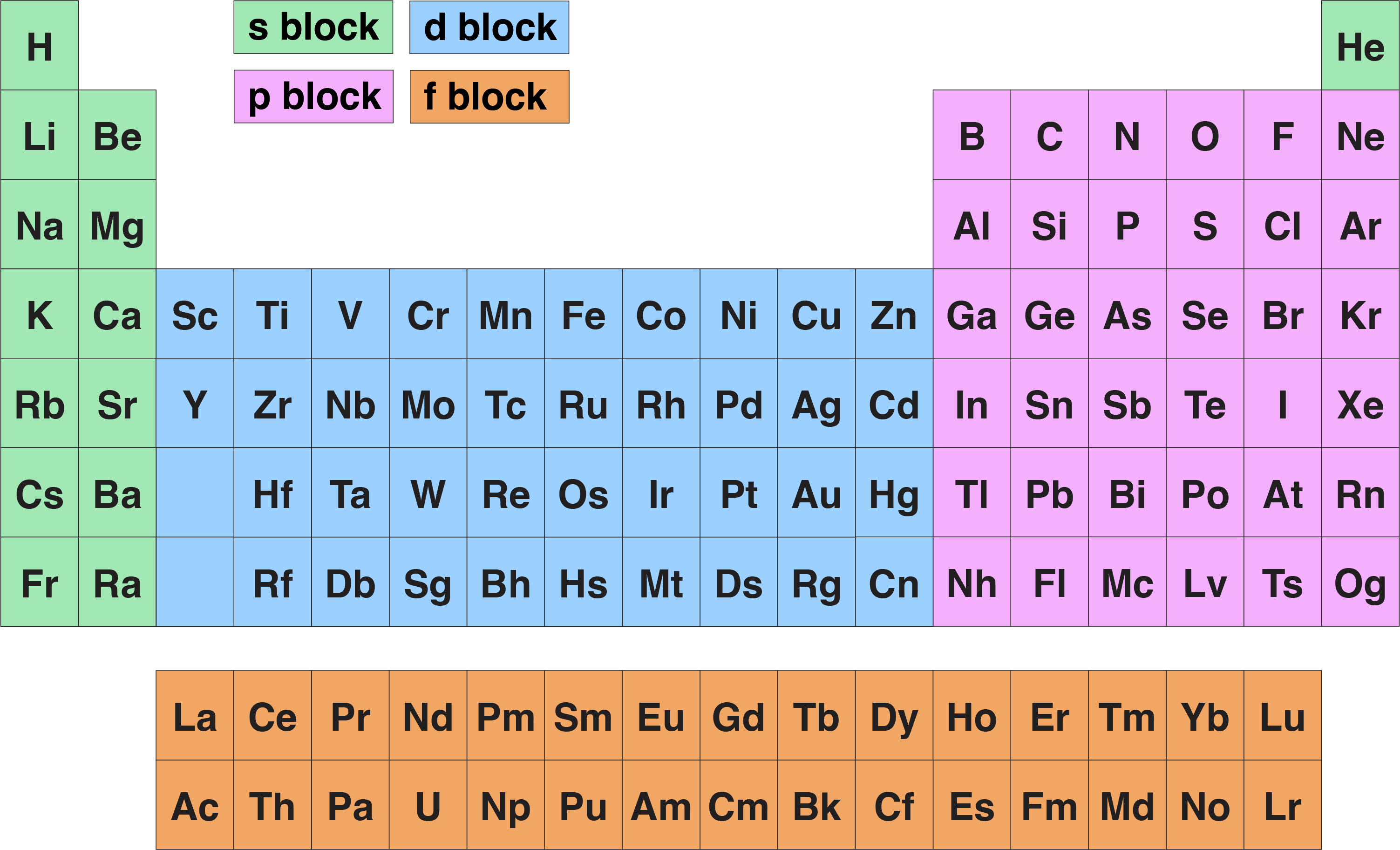
Periodic table
Shows all the elements arranged in rows and columns .
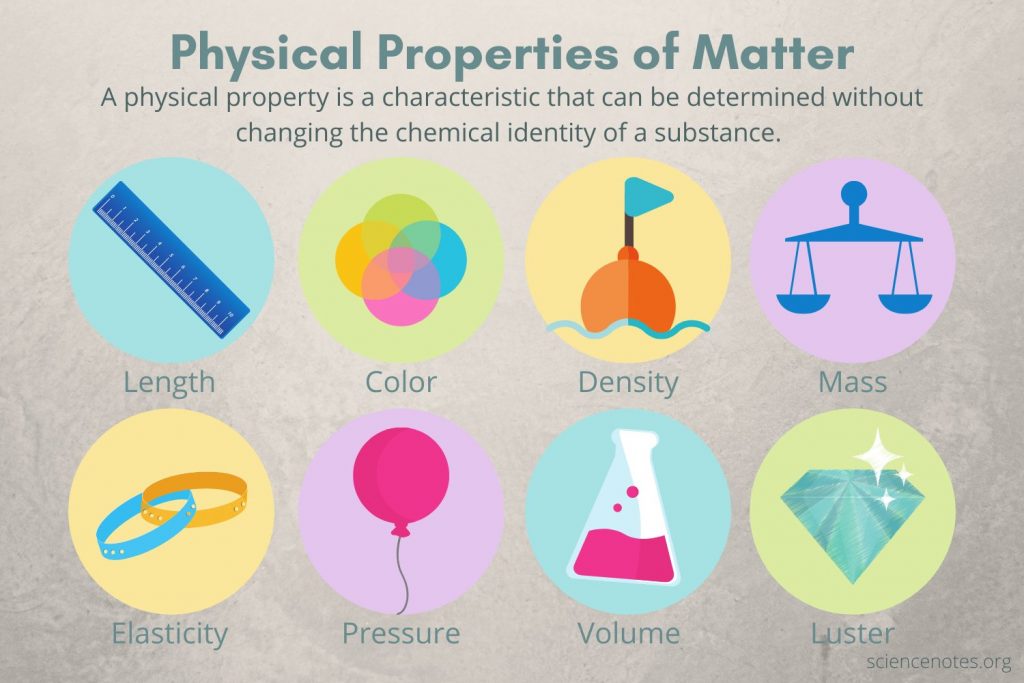
Physical properties
Features of a substance that can be observed without changing the substance itself .
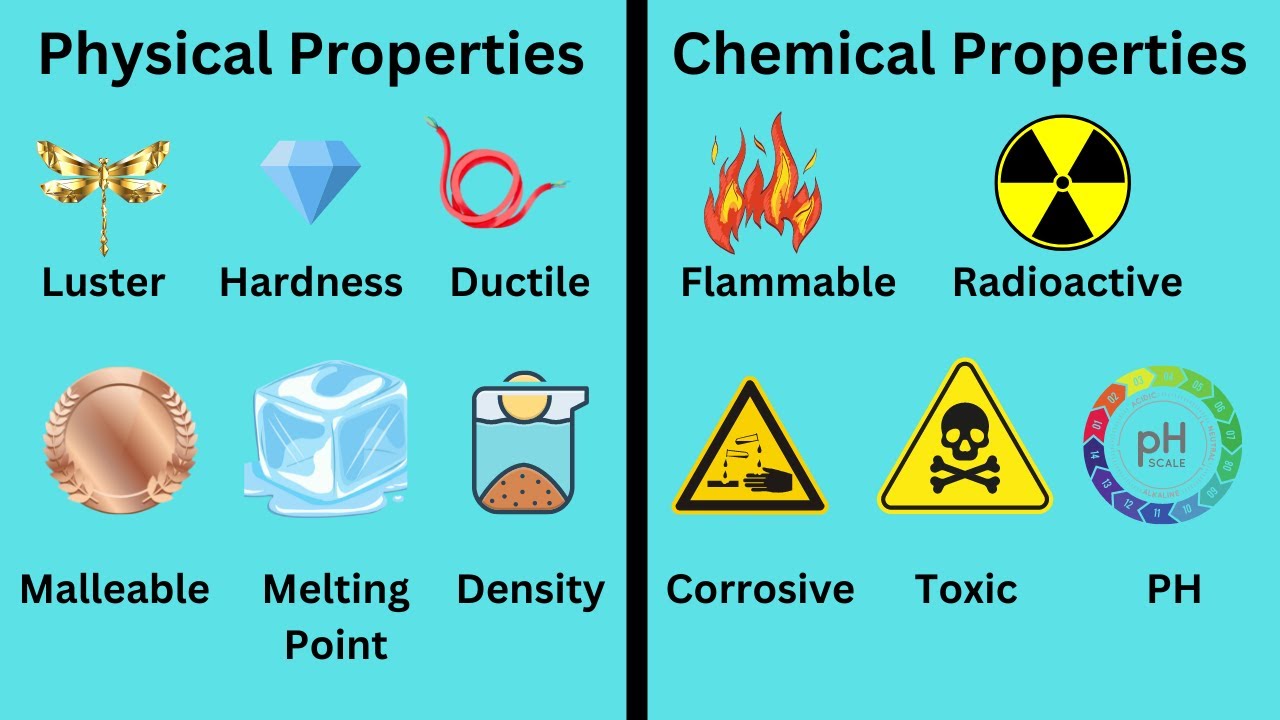
Chemical properties
Features of the way substance reacts with other substances.
Groups
Columns of the periodic table.
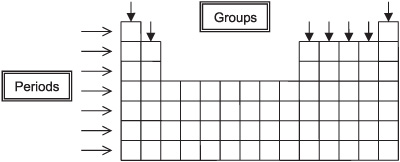
Periods
Rows of the periodic table.

Lustrous
Shiny when cut , scratched or polished .

Malleable
Easily bent or shaped .

Ductile
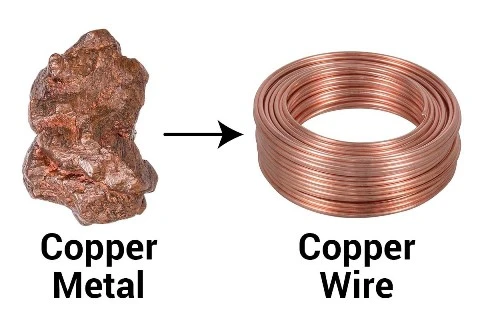
Can be turned into wires.
Number of protons:
Protons = atomic number
Number of electrons :
Electrons = Protons = atomic number
Number of neutrons :
Atomic mass - atomic number = neutrons
Atomic mass :
Atomic number + neutrons = atomic mass
Alkali metals
Found in group 1 of the periodic table.

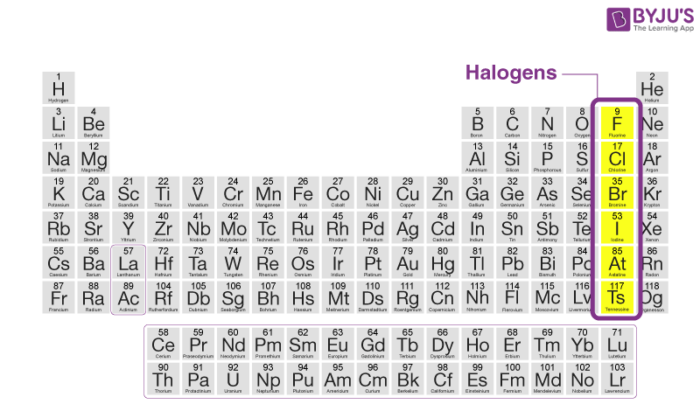
Halogens
Found in Group 7 of the periodic table.
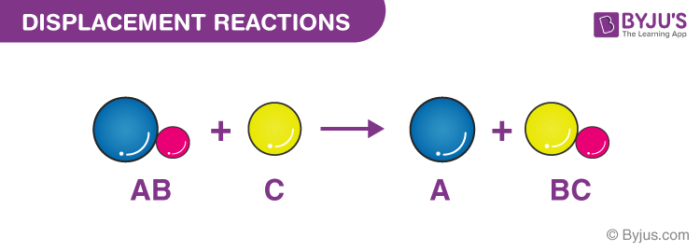
Displacement reaction
Occurs when a more reactive element displacements , or pushes out , a less reactive element from a compound that contains the less reactive element.
Compound
Two or more separate elements in a mixture.

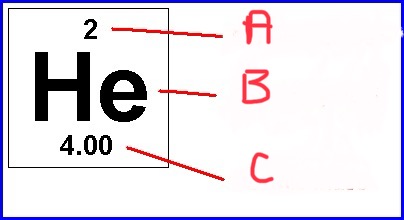
Name a b c


Who is Dmitiri Mendeleev?
He arranged the elements in order of increasing atomic mass .
He put elements into groups based on chemical properties.