Biology Unit 1 Topic 1: Structure of Water and Hydrogen Bonding
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Matter
Anything that takes up space and has mass (rocks, metal, oil, gases, organisms, etc. are all forms of matter).
Element
a substance that cannot be broken down into other substances by chemical reactions (92 elements occur in nature, periodic table).
Electronegativity
the measure of an atom’s ability to attract electrons to itself.
Atomic Number
the number of protons in an element (seen above the element symbol).
Atomic Mass
number of protons plus neutrons averaged over all isotopes.
Octet Rule
elements will gain, lose, or share electrons to complete their valence shell and become stable (like noble gases).
Chemical Bonds
an attraction between two atoms, resulting from the sharing or transferring of valence electrons.
Compounds
a substance consisting of two or more different elements combined in a fixed ratio (H2O and NaCl).
Covalent Bonds
when two or more atoms share electrons (usually between two nonmetals), forms molecules and compounds. (two types: nonpolar and polar)
Nonpolar Covalent Bonds
electrons are shared equally between two atoms (ex. O2)
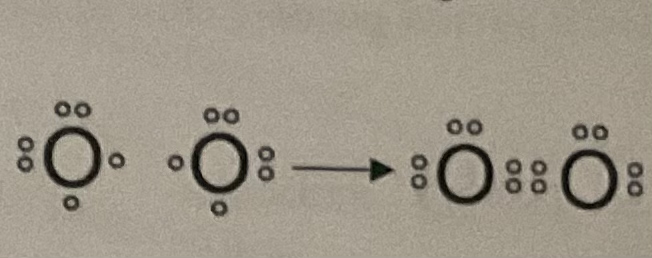
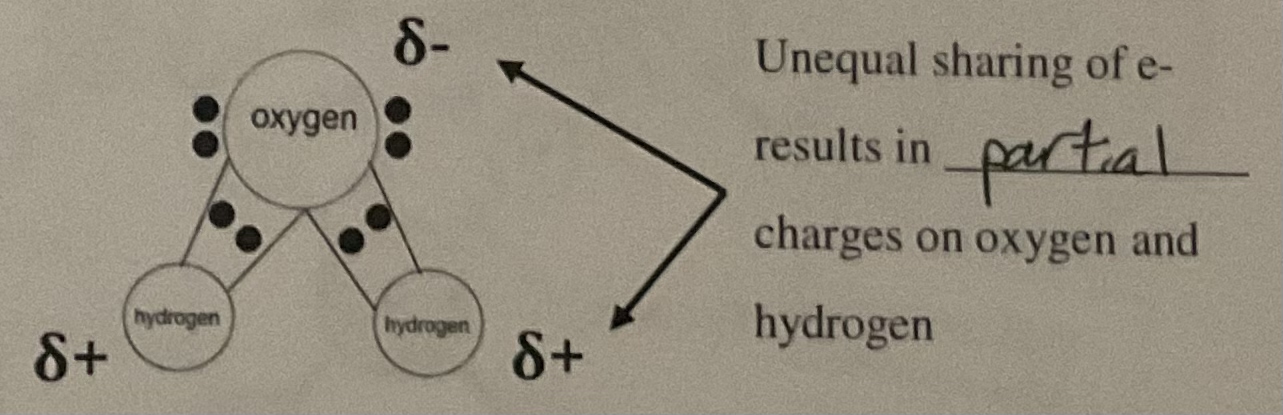
Polar Covalent Bonds
electrons are not shared equally between two atoms (ex. H2O)
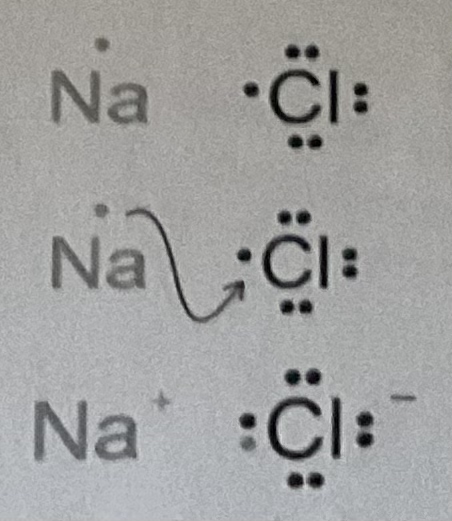
Ionic Bonds
the attraction between oppositely charged atoms (ions); usually metal and nonmetal (metal transfers electrons to nonmetal). Forms ionic compounds and salts (example: NaCl and LiF). (Cation: pos, Anion: neg)
Adhesion
the attraction to other molecules that are polar or have charge (H2O to other molecules).
Cohesion
attraction of molecules for other molecules of the same kind (H2O sticks together)
Capillary Action
the upward movement of water due to the forces of cohesion, adhesion, and surface tension (occurs when adhesion is greater than cohesion).
Solvent
dissolving agent in a solution
pH
a measure of how acidic or basic (alkaline) a solution is.
Acid
substance that releases hydrogen ions (H+) when dissolved in water.
Base
substance that accepts H+ or releases hydroxide ions (OH-)
Buffer
a solution that resists changes in pH when an acid or base is added (they maintain pH stability in biological systems).
Identify the elements that make up nearly all living matter.
Carbon, Hydrogen, Oxygen, Phosphorus, and Nitrogen (CHOPN)
How are hydrogen bonds different from other types of bonds?
The partially positive hydrogen atom in one polar covalent molecule is attracted to an electronegative atom in another polar covalent molecule.
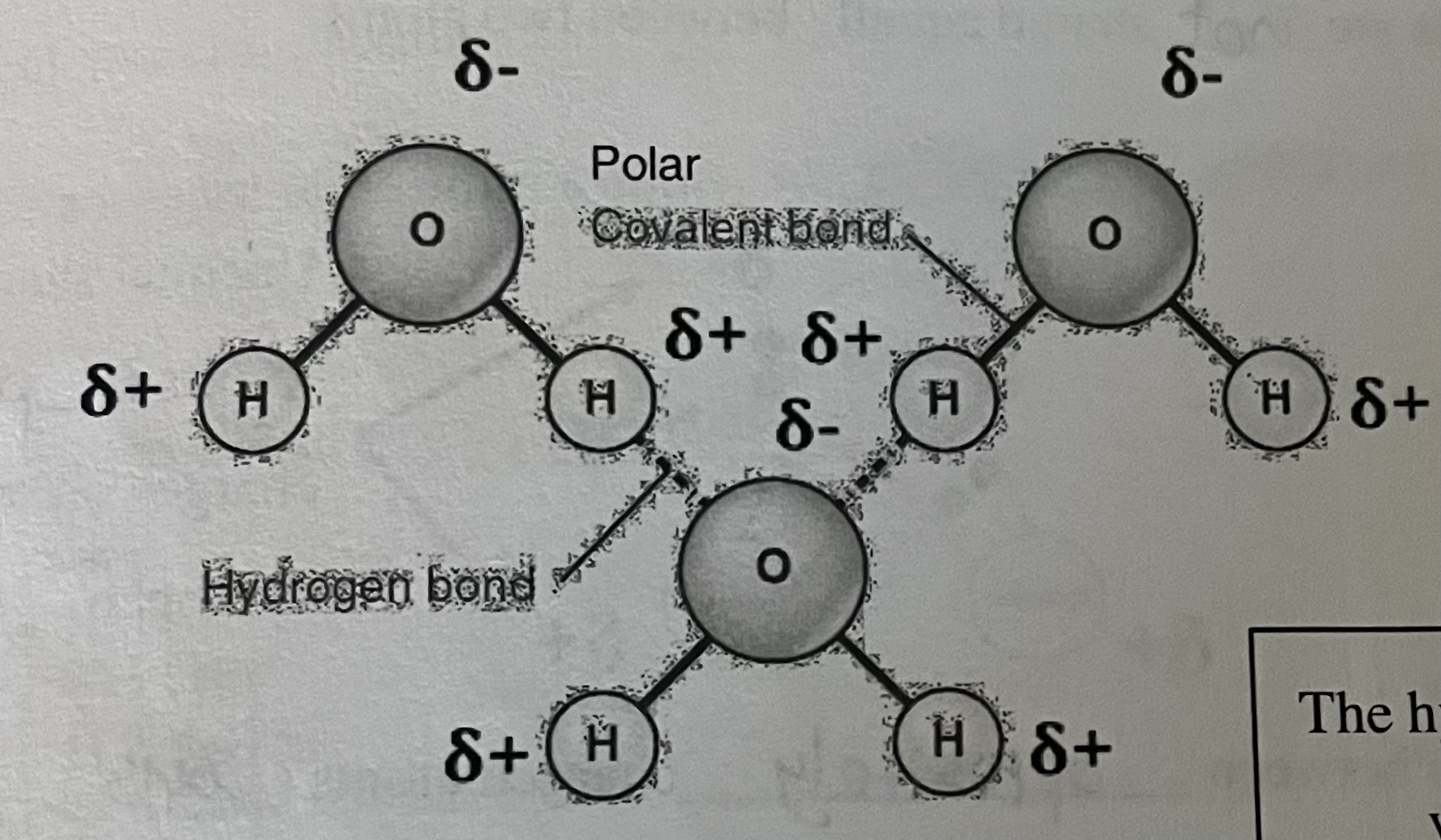
Draw a few water molecules. Label the types of bonds found in and between the molecules.
How does electronegativity affect the interactions between water molecules?
Causes hydrogen to have a partial positive charge and electronegative atom partial negative charge.
Imagine if O and H had the same electronegativity, what would that do to the properties of water?
O and H would not be attracted to each other in an intermolecular bond through the hydrogen bond and water would not be as structured.
Describe the properties of water; give an example of each.
Polarity: polar covalent bonds created by unequal sharing of electrons between oxygen & hydrogen within the molecule of water
H2O
Cohesion: hydrogen bonds between H2O molecules held together & increase cohesive forces
allows for transport of water & nutrients against gravity in plants
Adhesion: attraction of other molecules that are polar or have a charge
water can cling to the stiff walls of plants to resist the downward pull of gravity
Capillary action:upward movement of water due to cohesion, adhesion and surface tension
transport of water and nutrients in plants as water movies upwards
Temp Control: has high specific heat to resist changes in temp, high vaporization water requires large amt. of energy to evaporate
stabilizes ocean temp as water absorbs heat in daytime and releases it at night
Density (floating ice): water solidifies, expands, and becomes less dense
keeps environmental climate stable as it reflects the rays of the sun with the brightness of the ice
Solvent: polar molecules are attracted to ions and other polar molecules (form hydrogen bonds)
.
pH and buffering: water dissociates into hydrogen and hydroxide ions
acid substances release hydrogen ions when dissolved
bases accepts hydrogen or releases hydroxide
buffer resists changes in pH when acid or base is added
maintains pH stability in biological systems
Describe two ways in which the properties of water benefit organisms.
temperature control: evaporative cooling stabilizes earth’s climate & allows them to resist changes in on internal temp & overheating (sweating)
Density (floating ice): allows marine life to survive under floating ice sheets