bio biodiversity evolution and disease
1/139
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
140 Terms
plant passive defences physical
waxy cuticle, cellulose cell wall, stomata, bark, casparian strip
passive plant defences chemical
toxic compounds e.g catechol, bark resin, enzyme inhibitors like tannins, yeast on leaf surface
active plant defences
hypersensitivity- rapid death of tissue surrounding infection
reinforced cell walls (callose and lignin deposition)
plasmodesmata narrowing by callose
cytoplasm of nearby cells grows into xylem to make a callose wall
sieve pores of phloem blocked up by callose so sap can’t be transported
cell signalling in plant defence
when pathogens break down cell walls with cellulase and molecules produced act as signals to cell surface receptors. release phytoalexins
signalling molecules in plant defence
phytoalexins, salicylic acid, ethylene
what do phytoalexins do in plant signalling
disrupt pathogen metabolism, delay pathogen reproduction, disrupt bacterial cell surface membranes, stimulate the release of chitinase
role of salicylic acid in plant signalling
migrates through plant to unaffected areas and activates defence mechanisms that protect the plant against pathogens for a period. long-term protection called systemic acquired resistance
what does ethylene do in plant signalling
plants secrete it when pathogens are attacking. it vaporises and stimulates other leaves on the plant to react and other plants
primary non specific animal defences
skin/sebum, expulsive reflexes, mucous membranes, lysozymes, stomach acid
lysozymes role and where found
break down bacterial cell walls. found in blood, sweat tears, breast milk
what is commensal bacteria and where is it found
non-pathogenic bacteria found ina nd on humans. E. coli and Candida albicans are common. found in skin, mouth, and intestines.
why is commensal bacteria good
compete with pathogenic microorganisms for nutrients and space and prevent them from invading host tissue
second line of defence molecules
phagocytes and antimicrobial proteins
second line of defence responses
blood clotting, inflammation, wound repair, phagocytosis
blood clotting cascade overview
platelets trigger a reaction cascade that results in the formation of fibrin which forms a scab
the inflammatory response charecteristics
redness, swelling, heat, pain, loss of function
what is inflammation
a local repsonse to infection and tissue damage
what happens in the inflammatory response
mast cells secrete the cell signalling molecule histamine, which causes vasodilation (increases blood flow through capilliaries) and fluid enters the tissues, creating swelling. some plasma leaves the blood and phagocytes, and cells release cytokines that trigger an immune response in the infected area
what are cytokines
cell signalling compounds that stimulate inflammation and an immune response. they are proteins
what are interleukins
a type of cytokine. IL-1 and IL-6 promote inflammation, IL-1 targetting the brain causing drowsiness and fever.
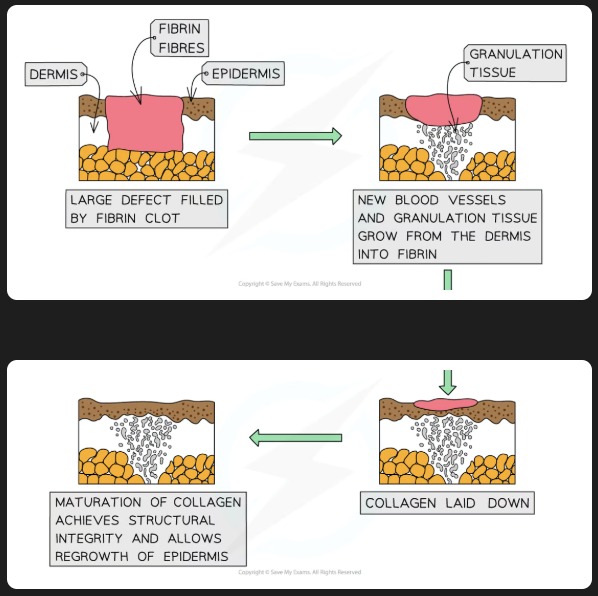
process of wound repair
scab is formed from blood clotting, stem cells divide by mitosis underneath. blood vessels form, collagen is produced, granulation tissue forms to fill the wound, stem cells move over the new tissue and produce epithelial cells, contractile cells cause wound contraction, unwanted cells die
what is a phagocyte
white blood cells produced in bone marrow.
phagocyte function
remove dead cells and pathogens. non-specific immune response
types of phagocyte
neutrophil, macrophage, dendritic cells
what is phagocytosis
the process of recognising and engulfing cells or particles
neutrophil definition
short lived phagocytes that act in the body tissues. multilobed nucleus. released in large numbers during infection.
neutrophil mode of action
attracted to pathogens or cells under attack by chemotaxis. recognise antibody molecules on pathogens and attatch to them. cell surface membrane extends out and around the pathogen, engulfing it and trapping the pathogen in a phagocytic vacuole. endocytosis. lysosome fuses to form phagolysosome, killing and digesting the pathogens, dying themselves.
what is a sign of dead neutrophils
pus
what is a macrophage
large long-lived phagocytes.
where are macrophages produced
bone marrow
where are neutrophils produced
bone marrow
what do macrophages mature from
monocytes, developing into macrophages when they leave the blood
where do macrophages reside
lungs, liver, spleen, kidney, lymph nodes
macrophages mode of action
initiate the specific immune response. don’t completely destroy pathogens, but take the antigens and become antigen presenting cells, whihc can be recognised by lymphocytes
what are dendritic cells
large phagocytic cells with lengthy extensions. large surface area.
where are dendritic cells found
throughout the body
what is the role of antigen presenting cells
t cell activation to provide a specific immune response
what can an antigen presenting cell present
the antigens from toxins, foreign cells and ingested pathogens
what are cytokines
cell signalling proteins produced by mast cells in damaged tissue, which attract white blood cells to the site of damage.
what are opsonins
chemicals that bind to and tag foreign cells, making them easily recognisable to phagocytes
what is a phagosome
a vesicle that forms within a phagocyte
what is a lysosome
vesicles that contain enzymes capable of breaking down biological polymers
what cells are involved in the cellular response
T cells (helper, killer, cytotoxic, regulatory, memory)
where do t cell mature
the thymus gland
where to b cells mature
the bone marrow
where do t lymphocytes orginate from
bone marrow
what happens when t cells mature
gain specific cell surface receptors called t cell receptors, which have a similar structure to antibodies
t cell role in immune response
complementary t cells binds to antigen presenting cell, and divide by mitosis to produce clones, which differentiate into t helper and t killers
what is a t helper cell
a t cell that release interleukins that increase phagocyte activity and activate b cells
what is a t killer cell
search for antigen presenting cekks and attatch to the antigens and secrete toxic substances to kill infected cells and the pathogen inside. secrete perforins
what is a perforin
a glycoprotein responsible for pore formation in cell membranes
what is a t memory cell
a t cell that remains in the blood after infection ready to be activated for clonal selection if there is reinfection
what is clonal selection
specific b or t lymphocytes containin receptors complementary to a particular antigen are selected and activated
what is clonal expansion
the process of rapid cell division resulting in the multiplication of genetically identical cell clones from a single parent cell
where are b lymphocytes found
the whole body, concentrated in the lymph nodes and spleen
what produces the humoral response
b lymphocytes
b cell activation
b cells with complementary antibody receptors bind to antigen presenting cells. clonal selection. also binds with interleukins and t helper cells
clonal expansion in b cells
activated b cells divide by mitosis to produce clones. some differentiate to plasma cells and some memory cells
plasma cell
a type of b cell that mass produces antibodies. short lived
primary immune response
response to a newly encountered antiged. time dela weaker and more short lived
secondary immune response
response to a previously encountered antigen. stronger more long lasting
what happens in the primary immune response
clonal selection and expansion of specific t and b cells, synthesis of antibodies
how long doe sthe primary immmune response take to produce antibodies
10-17 days
why is the seocndary immune resposne quicker
more memory cells present to be selected than the original clone than before so more antibodies can be quickly produced
what do b memory cells divide into
plasma cells and more memory cells
types of t memory cells
memory helper t cells, memory killer t cells
what are antibodies
globular glycoproproteins called immunoglobulins
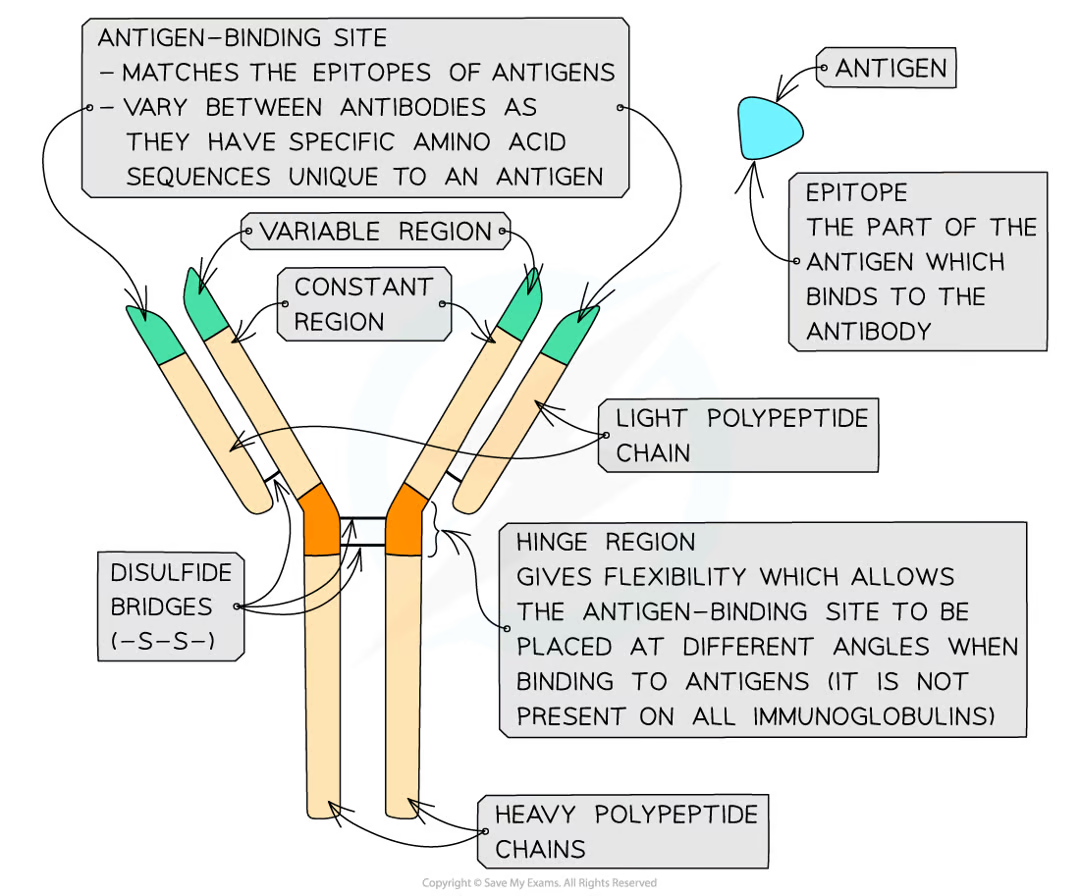
what structure do antibodies have
quaternary. y shape. two heavy polypeptide chains disulfide bonded to two light polypeptide chains. constant and variable region
how many types of mammalian antibody are there
5
what is the constant region of an antibody for
binding to b cells
what is the variable region of an antibody for
binding to specific antigens to form antigen-antibody complex
what is the hinge region of an antibody
gives felxibility to the antibody and allows antigen bindign sites at different angles
antibodies function
destroy pathogens directly or by activating other immune cells. anti-toxins, opsonins, agglutinins
antibodies as antitoxins
bidning to toxins produced by pathogens neutralising them
antibodies as opsonins
attatch to pathogens, making them easily identifiable to phagocytes. called opsonisation
antibodies as agglutinins
cause pathogens with antigen antibody complexes to clump together. called agglutination. makes phagocytosis easier
antibodies deactivating pathogens
attatch to bacteria flagella making them less active. phagocytosis easier
antibodies stimulating cell lysis
work with complement protein to cause holes in cell wall and lysis
active natural immunity and example
immune system naturally exposed to pathogen and produces antibodies against it. eg catching a cold
active artificial immunity example
vaccination
active immunity
antigen enters the body triggering a specific immune response
passive immunity
antibodies not produced by infected person
passive natural immmunity example
antibodies passed from mother to child through breastmilk
passive artificial immunity example
injection of antibodies or tetannus antitoxin
what is an autoimmune disease
the immune system attacks one or more self-antigens. targeted towards a single organ or the entire body
rheumatoid arthritis
autoimmune disease that solely affects the joints. begins int eh fingers and hands, spreading to shoulders and elsewhere.
rheumatoid arthritis symptoms
muscle spasms, inflamed tendons, lethargy, constant joint pain
causes of autoimmune disease
not fully understood. inheritable
herd immunity
indirect protection from an infectious disease when a population is immune through vaccination or previous infection
ring vaccination
when a person gets an infection, those in close contact are vaccinated against it
what could a vaccine contain
dead or inactivated pathogens, attenuated pathogen strains, harmless version of toxin, isolated antigens, genetically engineered antigens
what is an attenuated pathogen
a weakened version
why are booster vaccines given
to provide longer lasting immunity as memory cells do not last forever
who has weak immmune systems
babies elderly immunocompromised
antigenic variability
some pathogens have rapidly mutating antigens so a vaccine against one strain may not recognise the antigens from another strain
what are antibiotics
drugs that kill or inhibit bacterial growth
ways that antibiotics can affect bacteria
preventing the synthesis of dna, proteins, or bacterial cell walls
disrupting proteins activity int he cell membrane or enzyme action
why don’t antibiotics work on viruses
viruses don’t have cell structure so antibiotics cannot target metabolic reactions. also viruses are inside host cells
what is antibiotic resistance
what occurs when the use of antibiotics leads to resistant strains in bacteria due to mutations
how does antibiotic resistance occur
natural selection. variation in a population, antibiotic used and resistant survive, go on to reproduce and offspring have resistance too