Transport of oxygen by haemoglobin
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
cooperative binding
first oxygen binds to haemoglobin, changing tertiary structure
this creates/uncovers another binding site
change in shape (of haemoglobin) allows more oxygen to bind easily
How does partial pressure of oxygen affect oxygen-haemoglobin binding?
1.As partial pressure of oxygen increases, the affinity of haemoglobin for oxygen also increases
-so oxygen binds tightly to haemoglobin.
2.When partial pressure is low:
-oxygen is released from haemoglobin.
Bohr effect OxyHb graph
When a high carbon Dioxide concentration causes the oxyhemoglobin curve to shift to the right
The affinity for oxygen decreases b/c the acidic carbon dioxide changes the shape of haemoglobin slightly
Bohr effect
As partial pressure of carbon dioxide increases, the conditions become acidic due to the increase in H+ ions, causing haemoglobin to change shape.
The affinity of haemoglobin for oxygen therefore decreases
so oxygen is released from haemoglobin.and so more oxygen can be delivered to cells for respiration
How does saturation of haemoglobin with oxygen affect oxygen-haemoglobin binding?
It is hard for the first oxygen molecule to bind but once it does:
first oxygen molecule binds to haemoglobin, changing the tertiary structure
this reveals/uncovers another binding site
it changes the shape of haemoglobin to make it easier for the second and third oxygen molecules to bind
known as positive cooperativity.
It is then slightly harder for the fourth oxygen molecule to bind
because there is a low chance of finding a binding site.
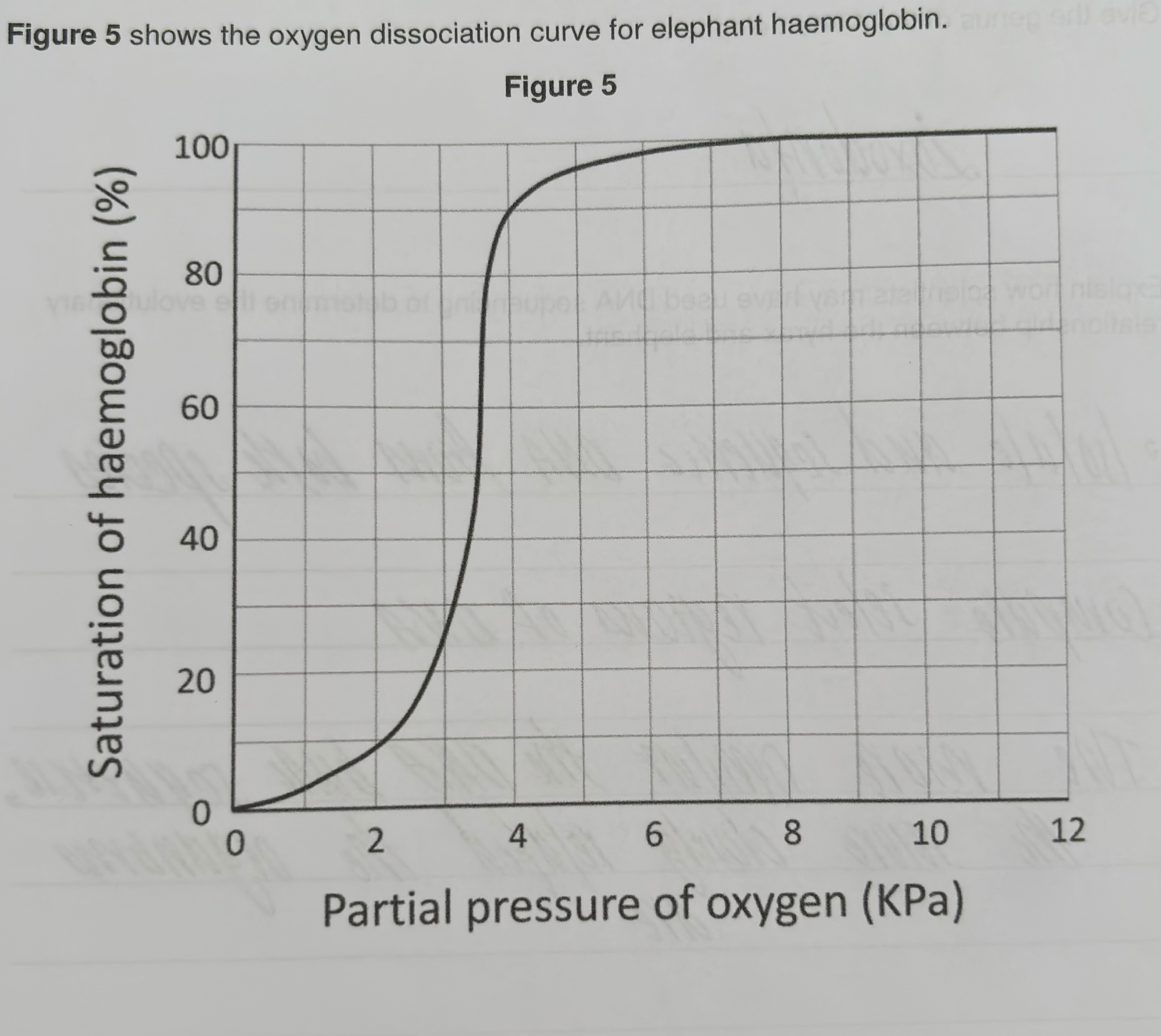
Describe and explain the shape of the curve in
Explain why oxygen binds to haemoglobin in the lungs.
Partial pressure of oxygen is high.
Low concentration of carbon dioxide in the lungs, so affinity of Hb to oxygen is high.
Positive cooperativity
Explain why oxygen is released from haemoglobin in respiring tissues.
Partial pressure of oxygen is low
High concentration of carbon dioxide in respiring tissues, so affinity of Hb to oxygen decreases.
if oxyhaemoglobin dissociation curve goes towards the left
haemoglobin has higher affinity for oxygen
so it releases less oxygen/ uploads more oxygen
Dissociates oxygen more readily
it becomes saturated at lower partial pressure
if oxyhaemoglobin dissociation curve shifts to the right
haemoglobin has a lower affinity for oxygen
so it unloads/dissociates more oxygen, more readily into cells for respiration
therefore greater (rate of ) respiration
at a particular partial pressure, more oxygen released
Foetal haemoglobin
has higher affinity for oxygen (than adult haemoglobin), even at the same partial pressure
loads oxygen from mothers haemoglobin/blood
so more oxygen moves from the mother to the fetus
advantage of replacing fetal haemoglobin with adult haemoglobin
adult haemoglobin has a lower affinity for oxygen
so more oxygen is released and delivered to respiring cells
easier unloading of oxygen for aerobic respiration
how oxygen is loaded, transported and unloaded in the blood
haemoglobin has a high affinity for oxygen
at high partial pressure: oxygen is uptaken into the lungs
at low partial pressure: oxygen is released into respiring cells
this is due to higher CO2 conc. (bc respiration)
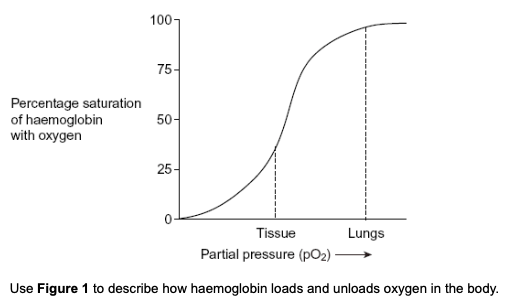
Figure 1 shows the oxygen dissociation curve for human haemoglobin.
loading of oxygen at high partial pressure
in the lungs, haemoglobin has a high affinity for oxygen
haemoglobin unloads oxygen at low partial pressure
Explain how the shape of a red blood cell allows it to take up a large amount of oxygen in a short time.
large SA:V ratio
for diffusion
thin
so oxygen can release all haemoglobin
Explain how oxygen in a red blood cell is made available for respiration in active tissues.
low pH due to increased CO2
increased dissociation of oxygen from haemoglobin
oxygen diffuses from rbc to tissues
What is meant by the term partial pressure?
the measure of concentration of a gas