Seperating, aqueous and reactions chemistry test
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
What are mixtures?
Mixtures are a combination of two or more substances that have not been combined chemically, meaning they can be separated by physical or mechanical means.
How do we separate mixtures?
The properties of the components determine what technique is used to separate the mixture.
The properties are: Particle size, solubility, magnetism, electrostatic attraction, boiling point and density.
What are the methods of separating by particle size?
Sieving: Larger particles remain in the sieve and the smaller ones will travel through (Liquid and solid/Solid and solid).
Filtration: Liquid (Filtrate) passes through filtration paper and solid (residue) remains. (Liquid and solid)
What are the methods of separating by boiling point?
Vaporisation: Retrieve a solid that was dissolved in a liquid. Solvent is converted into vapour leaving behind solute.
Boiling: Quicker process than evaporation where it is based on the large difference in boiling points of solute and solvent.
Distillation (simple): Used for separating two or more liquids or liquid from solids in a solution but retrieving the liquid component. Difference in B.P must be more than 50 degrees Celsius.
Distillation (Fractional): Used to separate liquids with similar B.P as mixture is heated and components rise up a fractioning column. It’s commonly used to separate crude oil.
What is the method of separating by density and solubility?
Separating funnel: Used to separate two immiscible liquids. The liquids separate into two layers as they are insoluble in each other.
What are the methods of separating by magnetism and electrostatic attraction?
Magnetic separation: Strongly magnetic materials are separated from low or nonmagnetic materials using a magnet.
Electrostatic separation: Separates particles based on electrical charge. Differently charged particles will be attracted or repelled.
What is Mass spectrometry?
An instrument used to separate particles with different masses and measure the mass and the relative proportions of these particles in a mixture.
What are the important steps of the mass spectrometry?
-Ionisation
-Acceleration
-Deflection
-Detection
What is ionisation in mass spectrometry?
The atom is ionised by knocking one or more electrons off to give a positive ion. Mass spectrometers always work with positive ions.
What is acceleration in mass spectrometry?
The ions are accelerated so that they all have the same kinetic energy.
What is deflection in mass spectrometry?
The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected. Also the more the ion is charged, the more it gets deflected.
What is detection in mass spectrometry?
The beam of ions passing through the machine is detected electrically.
How does an atom reach an excited state?
If the atoms are given sufficient energy, electrons will absorb the exact amount of energy required to jump to a higher energy level (electron shell). The excited electrons very quickly return to their ground state, releasing the absorbed energy, in the form of light.
What is the absorption spectra?
•A series of dark lines superimposed on a continuous spectrum.
•The dark lines show the wavelengths of light that have been absorbed
What is the emission spectra?
•A series of bright lines against a black background.
•The bright lines indicate the wavelengths of light that have been emitted.
How can we identify elements according to the colour emission?
The amount of energy absorbed or emitted is the same for the same atom but is specific to that atom and thus allows the identification of atoms in a sample
What is the flame test?
Metals will produce distinctive flame colours when their salts are vaporised in a flame.
This is caused by the atom absorbing energy from the flame, causing the electrons to become excited. The electrons then ‘fall back down’ releasing the energy in the form of visible light that is seen in the flame.
This allows the identification of the substance.
What is the AAS?
An AAS is used in industry, in research and in monitoring the purity of substances such as air, water, and food.
AAS is a quantitative technique that can be used to measure the concentration of metal ions in solution at very small concentrations (the trace level) in ppm or ppb.
What are the important parts of the AAS?
Nebuliser
Atomiser
Monochromator
Detector
Output
What is the nebuliser?
sucks up samples at a controlled rate, creates a fine aerosol spray and mixed the aerosol and fuel and oxidant thoroughly for introduction into the flame.
What is the atomiser?
the separation of particles into individual molecules and breaking molecules into atoms. This is done by exposing the anolyte to high temperatures in a flame or graphite furnace.
What is the monochromator?
Is used to select the specific wavelength of light with is absorbed by the sample and exclude other wavelengths.
What is the detector?
The light selected by the monochromators is directed onto a detector which converts the light signal into an electrical signal proportional to the light intensity.
What are the forms of an output?
This may be in many forms, a printout, on the screen or a meter.
What is chromatography?
the physical separation of a mixture into its individual components. We can use chromatography to separate the components of inks and dyes, such as those found in pens, markers, clothing, and even candy shells. Chromatography can also be used to separate the colored pigments in plants or used to determine the chemical composition of many substances.
What are the two purposes of chromatography?
Analytical and preparative.
What is the analytical purpose of chromatography?
Determines chemical composition of a sample.
What is the preparative purpose of chromatography?
Collect and purify components of a sample.
What is the mobile phase?
Fluid used to carry the components of a mixture up or along the stationary phase so they can separate.
What is the stationary phase?
Immobile surface that different components of a mixture can adhere to when carried along it by the mobile phase in chromatography.
What is the retardation factor?
Distance travelled along the stationary phase by a particular component of a mixture relative to the distance travelled by the solvent. (TLC)
What is retention time?
How long a particular component of a mixture takes to exit the column. (GC and HPLC)
How does chromatography work?
•As the mixture is swept along with the mobile phase, some of its components are adsorbed more strongly to the surface of the stationary phase than others.
•They will eventually desorb and move on.
•This process of differential adsorption and desorption leads to the components travelling at different speeds so they separate out.
What is thin layer chromatography(TLC)?
•The substances to be separated are placed on the plate and this plate is then placed in a container with a solvent.
•Stationary phase is the specially coated glass, plastic or metal plate.
•Coating is an adsorbent material such as silica gel, fine alumina or powdered cellulose.
•Mobile phase is a solvent of some type, depending on the substances to separate – maybe polar or non-polar.
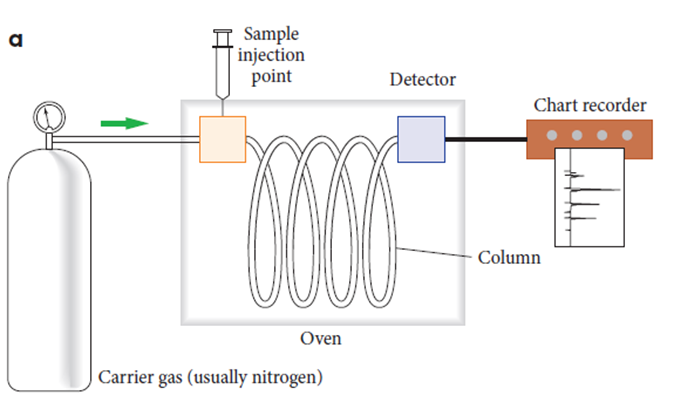
What is gas chromatography(GC)?
•The substance to be analysed is either a gas mixture or a substance that can be vaporised.
It is suitable for molecular substances with a Mr less than 300 and can be vaporised without damage to their molecules.
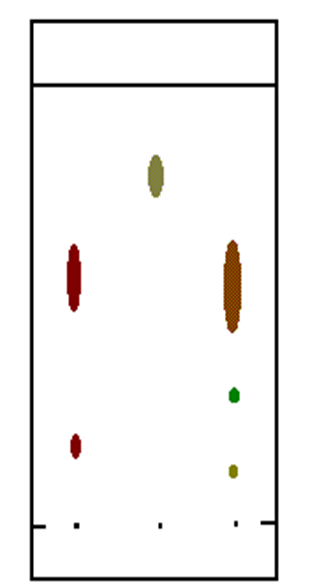
What does the number of peaks in GC output tell us?
•The number peaks tell how many individual components were in the mixture.
•The retention time tells what the component is.
•The relative areas under the peaks can be used to determine the relative proportion of each component in the substance, BUT to find the actual concentration of the component standards need to be prepared and a calibration curve made.
What are the uses of chromatography?
•Detection of drugs and explosives in airline baggage.
•Air monitoring in polluted environments
•Urine testing of athletes
•Horses and greyhounds for illegal drugs
What is high performance liquid chromatography?
•Similar to GC except the mixture analysed remains as a liquid and it is moved through the instrument by a liquid instead of a carrier gas.
•HPLC can be used for substances with Mr over 300 and substances that decompose when heated.
•HPLC can also be used to separate and purify substances.
What do the outputs in HPLC tell us?
As with GC the position of the peak can be used to identify the presence of a component once standards are put through the machine but a calibration curve needs to be done to determine the concentration.
What are the uses of HPLC?
•Contamination of foods
•Purification and analysis of proteins and peptides
•Cyanide analysis
•Analysis of toxins in seafood
•Pharmaceutical analysis
•Food analysis during production
What is ionisation?
The process whereby a molecular substance interacts with water producing ions.
What is dissociation?
The process whereby an ionic substance dissolves in water producing separate ions.
What are saturated solutions?
A solution in which no more pure solute can be dissolved into a given mass of solvent at a specified temperature and pressure.
What are unsaturated solutions?
A solution in which more pure solute can be dissolved into a given mass of solvent at a specified temperature and pressure.
What are supersaturated solutions?
More solute has been added than can be dissolved in a given mass of solvent at a particular temperature and pressure.
What is a non electrolyte?
These substances when dissolved in water do not produce any ions.
Does not conduct electricity
Any substance that does not fit into the other categories.
What is a weak electrolyte?
When a substance is dissolved in water and only partly dissociates or ionises and thus is only partly present in ions.
Only conducts a small amount of electricity
This is indicated by a double arrow (⇌)
Weak acids and bases are weak electrolytes.
What is a strong electrolyte?
When a substance is dissolved in water and it completely ionises or dissociates and is thus present entirely as ions.
Good conductor of electricity
This is indicated by a one way arrow (à).
Strong acids, strong bases and ionic compounds (that are soluble) are strong electrolytes.
What are the properties of acids?
Tastes Sour
Conduct Electricity
Corrosive, which means they break down certain substances. Many acids can corrode fabric, skin, and paper
Some acids react strongly with metals
Turns blue litmus paper red
What are the uses of acids?
Acetic Acid = Vinegar
Citric Acid = lemons, limes, & oranges. It is in many sour candies such as lemonhead & sour patch.
Ascorbic acid = Vitamin C which your body needs to function.
Sulfuric acid is used in the production of fertilizers, steel, paints, and plastics.
Car batteries
What are the properties of bases?
Feel Slippery
Taste Bitter
Corrosive
Can conduct electricity. (Think alkaline batteries.)
Do not react with metals.
Turns red litmus paper blue.
What are the uses of bases?
Bases give soaps, ammonia, and many other cleaning products some of their useful properties.
Chalk and oven cleaner are examples of familiar products that contain bases.
Your blood is a basic solution
What is an Arrhenius acid?
A substance that produces H+ (H3O+) in water.
What is an Arrhenius base?
a substance that produces OH- in water.
What are strong acids? (examples)
Completely ionises in water
Conduct electricity
Strong electrolytes
Examples: Nitric acid, Sulfuric acid, Hydrochloric acid, and Bromic acid
What are weak acids? (examples)
Only partly ionises in water
Conduct little to no electricity
Weak electrolytes
Examples: Acetic acid, Hydrofluoric acid, Phosphoric acid, and Carbonic acid
What are strong bases? (examples)
Completely dissociate in water
Conduct electricity
Strong electrolytes
Examples include: Group 1 and 2 hydroxides and oxides
What are weak bases? (examples)
Partially ionise in water
Only conduct a little electricity
Weak electrolytes
Examples: Ammonia, Group 1 and 2 carbonates and Group 1 and 2 phosphates
What is a monoprotic acid?
An acid that produces only 1 Hydrogen that can be ionised.
What is a polyprotic acid?
An acid that produces more than 1 hydrogen ion in solution.
Ionises in sequential steps.