introduction to chemistry
1/37
Earn XP
Description and Tags
all about potential (G)
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
what was the great oxygenation event?
cyanobacteria filled the atmosphere with oxygen
what type of atmosphere (reducing/oxidizing) did earth used to have? what does this mean?
reducing; earth used to have an abundance of hydrogen gas
what type of atmosphere (reducing/oxidizing) does earth have now? what does this mean?
oxidizing; there are low levels of hydrogen gas and high levels of oxygen gas
rule of life (our cells + atmosphere) in terms of entropy.
our cells must actively work to maintain out low-entropy, highly ordered state by constantly consuming energy and increasing the entropy of our surroundings— build complex structures in exchange of releasing heat and waste
equilibrium vs steady state (definition & energy level)
equilibrium is a closed system state in which forward/reverse rates balance, requiring no net energy— minimum energy. steady state is an open system state with constant variables that need constant work/energy to maintain stable level— high energy.
higher potential energy reactants result in __ (higher/lower) potential energy products
lower (because energy all used up)
what does ∆G refer to in biology?
∆G refers to the change in potential energy
ionic bond vs covalent bond
ionic bond is when an atom transfers its electron to another atom (often a high potential energy to a low potential energy). covalent bond is when an atoms share electron(s).
rank electronegativity from high to low: carbon, phosphorous, hydrogen, nitrogen, oxygen, chlorine, potassium, sodium. Do electrons move spontaneously from left to right or right to left?
O (3.5) > Cl (3.1) > N (3.0) > C (2.5) > P(2.1) > H (2.1) > Na (0.9) > K (0.8). Electrons move spontaneously from left (high EN0 to right (low EN).
what does electronegativity refer to?
strongly pulls shared electrons towards itself in a bond
in sodium-chlorine bond, what has been reduced and what has been oxidized?
the sodium has been oxidized by chlorine because it lost an electron to chlorine and chlorine has been reduced by sodium because it gained an electron from sodium (reduced = got more negative)
can a covalent bond formation involve redox?
yes, but it’s a partial transfer of charge
Sort the following items in the order in which they occured:
i. Eukaryotes evolve
ii. Oceans present on Earth
iii. Collision of the Earth with the planetoid Theia
iv. Photosynthesis evolves
v. Cyanobacteria evolve a pathway to oxidize water
Collision of the Earth with the planetoid Theia, Oceans present on Earth, Photosynthesis evolves, Cyanobacteria evolve a pathway to oxidize water, Eukaryotes evolve
A chemical reaction occurs in a test tube in lab. During the reaction, "reactants" gradually become "products" until equilibrium is reached. At equilibrium:
a) Some molecules of reactant become product, and some molecules of product become reactant
b) no more reactions occur: no moleculted of reactant become product, and no molecules of product become reactant
c) delta G = 0
d) both a and c are true
d) both a and c are true (equal amounts of products and reactants formed a + there will be no change in potential energy b/c no energy has been exhausted)
In the reaction 2H2 + O2 --> 2H2O, what is being oxidized?
a) O2
b) There is no redox in this reaction
c) H2
d) H2O
c) H2 (oxidized = give away electrons in bond → hydrogen will give its electron(s) away and oxygen pull them closer)
In living things, the two major components of G, "Potential energy", are...
a) Gibbs Free Energy, and Work
b) Concentration and molecular structure
c) Concentration and dilution
d) Pressure and Volume
b) Concentration and molecular structure
A glucose molecule has a bond between O and C. This is a...
a) Ionic bond
b) Nonpolar covalent bond
c) Polar covalent bond
d) Hydrogen bond
c) Polar covalent bond (EN diff > 0.4)
∆Gº refers to the change in Gibbs free energy under (biological) standard conditions. These standard conditions include:
a) Temperature = 25 degrees Fahrenheit
b) The reaction is at equilibrium
c) pH = 0
d) All chemical components (reactants and products) are constantly at 1 Molar
d) All chemical components (reactants and products) are constantly at 1 Molar
H2O is a liquid at room temperature while CO2 is a gas. Why is this?
a) water molecules are attracted to each other due to their dipole moment, CO2 molecules are not polar
b) the polar covalent bonds in CO2 cause the molecules are not polar
c) water has a lower molecular weight than CO2
d) water has a higher molecular weight than CO2
a) water molecules are attracted to each other due to their dipole moment, CO2 molecules are not polar (these weak dispersion forces allow molecules to easily escape as gas)
The ingredients listed below will give you 2 ml of 1/2 molar sodium chloride after mixing. Which will release the greatest amount of energy (=has the largest negative ∆G) when doing so?
a) Mixing enough sodium metal and Cl2 gas to produce the amount of sodium chloride salt described above, with 2 ml water
b) Mixing dry NaCl (enough to make a 1ml, 1 molar NaCl solution), plus 2 ml water
c) Mixing two 1 ml tubes of 1/2 molar NaCl
d) Mixing 1 ml of 1 molar NaCl, plus 1 ml of water
a) Mixing enough sodium metal and Cl2 gas to produce the amount of sodium chloride salt described above, with 2 ml water (we want to look for exothermic reaction or reactant with high potential energy + equal amounts ml & molar start and end)
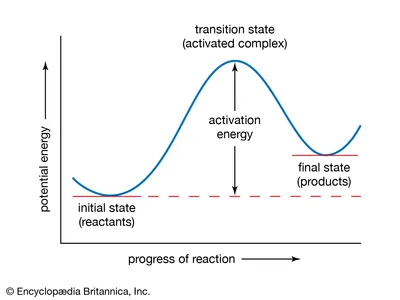
What is the difference between the transition state energy and the activation energy of a particular reaction?
a) The transition state energy is a constant, activation energy is not
b) Activation energy is the amount of energy in the structure of the transition state
c) Nothing. Transition state energy and activation energy are two terms for the same thing
d) Changes in delta Gact cause changes in delta Grx, but changes in transition state energy do not
a) The transition state energy is a constant, activation energy is not
what is the reducing agent: C3H8 + 5O2 —> 3CO2 + 4H2O + heat & light
C3H8 (propane)
(reducing and oxidizing agents will always be reactants. In this example, oxygen will pull electrons close and propane will donate its electrons. Since oxygen is being reduced, propane will be the reducing agent and the one that becomes oxidized.)
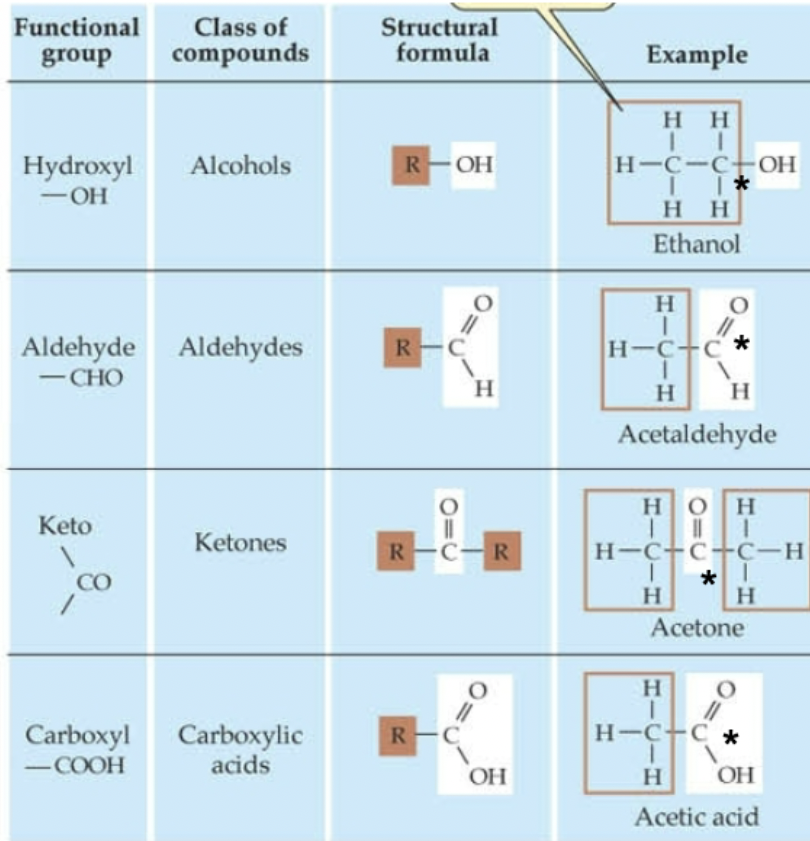
which C* is most oxidized?
carboxylic acid (due to presence of 2 oxygen (oxidizing agent) atoms)
does oxidation have to involve oxygen?
no, just a transfer of electrons (e.g. CO2 can be oxidizing agent)
what do chemical reactions provide in terms of entropy?
chemical reactions provide energy that allows life to reverse local entropy
methane and CO2 now vs past
Now, methane is fuel that is burned and CO2 is the waste product. In the past, methane was a waste product and CO2 was the fuel.
what is the sign of ∆G in an exergonic reaction?
negative (low potential energy - high potential energy)
when does a forward reaction take place (X + Y → Z)?
only when ∆G is negative
a positive ∆G of high magnitude
a. allows you to get lots of energy from a reaction
b. can be achieved by increasing the concentration of products
c. can be achieved by increasing the concentration of reactants
d. both A and B
d. both A and B (endothermic reaction & reverse reaction → reaction dependent of products)
what are 3 components of a good fuel
release energy when burned (-∆G)
more bang per ounce
only burn when needed/wanted
what is activation energy? is it constant?
activation energy is the extra energy required for a reaction to reach transition state and for it to actually occur. It is not constant.
what is transition state? is it constant?
transition state is the structure of a molecule during a reaction (peak). It is constant.
does the sign of ∆G dictate the rate of reaction? If not, what does it dictate?
no, the amount of energy released does not dictate the rate of the reaction. It dictates the net direction.
what do catalysts do in a reaction? Are they consumed? Do they affect ∆G? What does it affect?
Catalysts take and and give electrons, but are not consumed as they are regenerated in their original form at the end of the cycle. They do not affect ∆G since they are not consumed, but do affect the rate of reaction since they lower Ea.
what is our biological catalyst(s)?
enzymes
what are 2 ways life can manipulate ∆G?
1) manipulating concentrations
2) physically coupling endergonic reactions with exergonic reactions
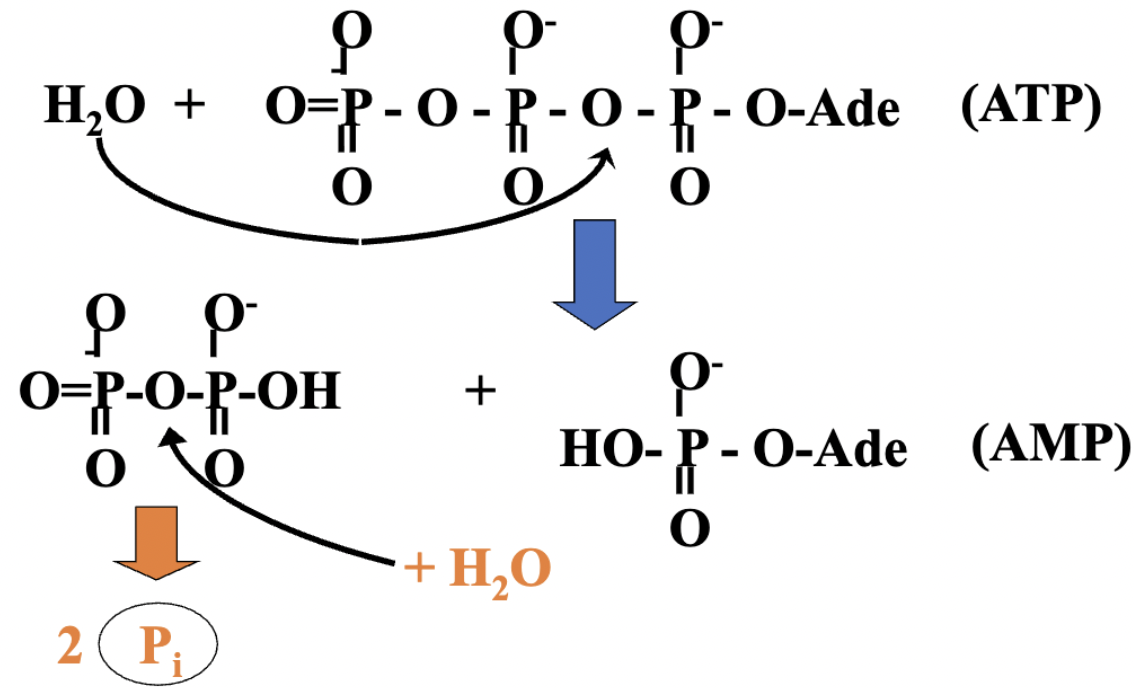
what occurs in the second reaction?
the second reaction pulls the first, and the cell keeps pyrophosphate [PPi] low to enhance the -∆G of ATP → AMP + PPi— double dose of energy
what type of carrier is ATP?
an energy carrier in which energy is carried until bonds break