Understanding the Three States of Matter
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Particle Theory
The theory that explains the behavior of particles in solids, liquids, and gases.
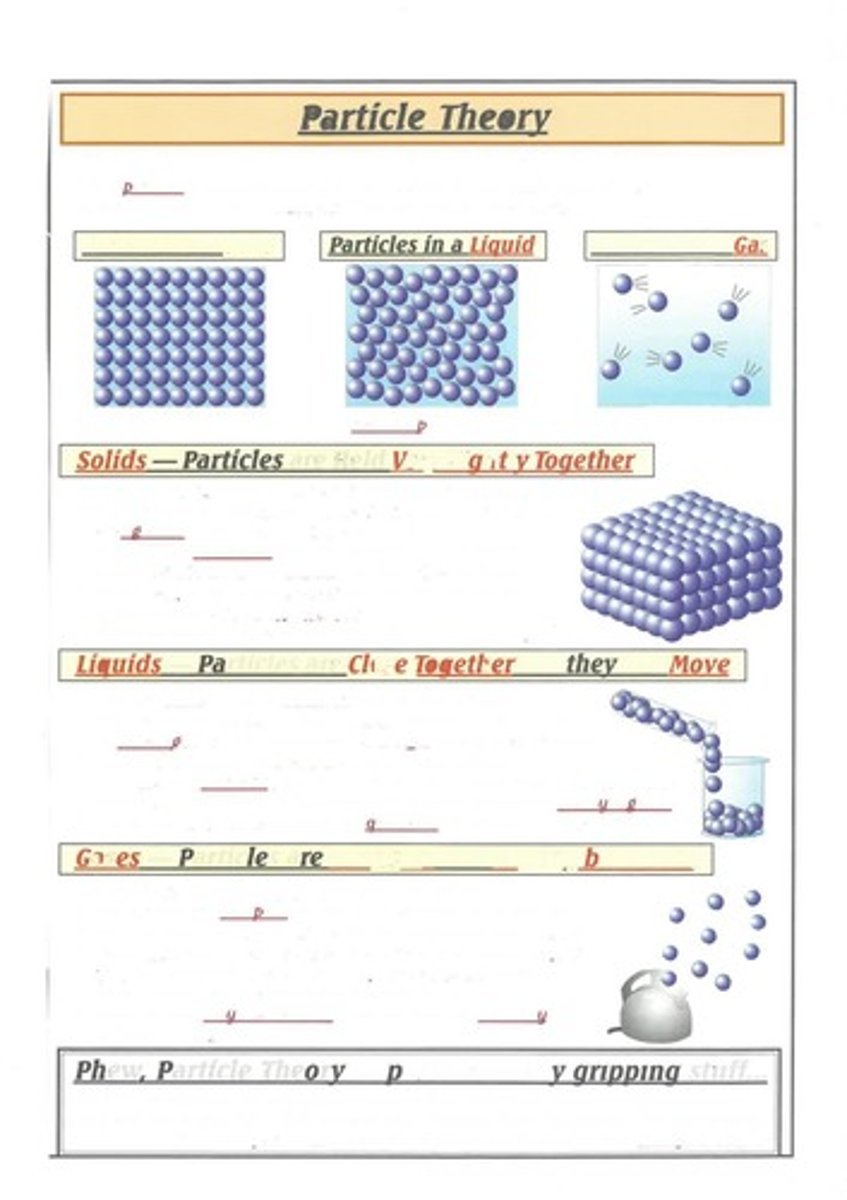
Particles in a Solid
Particles are held very tightly together with strong forces of attraction, fixed in a regular arrangement, vibrating but not moving from their positions.
Particles in a Liquid
Particles are close together but free to move past each other, constantly moving in all directions, and liquids do not keep a definite shape but have a definite volume.
Particles in a Gas
Particles are far apart, free to move quickly in all directions, and gases do not keep a definite shape or volume, expanding to fill any container.
Solids
Have a definite shape and volume, can't flow, and are not easily compressed.
Liquids
Have a definite volume, match the shape of their container, flow easily, and are not easily compressed.
Gases
Have no definite volume, always fill the container they are in, and are easily compressed.
Density of Solids
Solids are usually dense, as there are lots of particles in a small volume.
Density of Liquids
Liquids are quite dense, as there are quite a lot of particles in a small volume.
Density of Gases
Gases have very low densities because there are not many particles in a large volume.
Compressibility of Solids
Solids can't easily be compressed because the particles are already packed very closely together.
Compressibility of Liquids
Liquids won't compress easily because the particles are packed closely together.
Compressibility of Gases
Gases can be compressed easily because there's a lot of free space between the particles.
Arrangement of Particles in Solids
Particles are held closely in fixed positions in a very regular arrangement.
Movement of Particles in Solids
Particles vibrate to and fro but do not move from their positions.
Movement of Particles in Liquids
Particles are close but free to move past each other and are constantly moving in all directions.
Movement of Particles in Gases
Particles move fast, collide with each other and the container.
Three States of Matter
Materials come in three different forms: solid, liquid, and gas.

Properties of Solids
Solids have a definite volume, a definite shape, high density, and are not easily compressed.
Properties of Liquids
Liquids have a definite volume, match the shape of the container, have medium density, and are not easily squashed.
Properties of Gases
Gases have no definite volume, take the shape of the container, have very low density, and are easily squashed.
Forces of Attraction in Solids
There are strong forces of attraction between particles.
Forces of Attraction in Liquids
There are some forces of attraction between the particles.
Forces of Attraction in Gases
There are no forces of attraction between the particles.
Behavior of Substances
A property of a substance is a way of saying how it behaves.