TDL6 - Entropy
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
£rd law TD
The entropy of a perfect crystal is 0 at 0K
standard reaction entropy, ∆𝐫𝑆o is…
The difference in molar entropy between the products and the reactants in their standard states
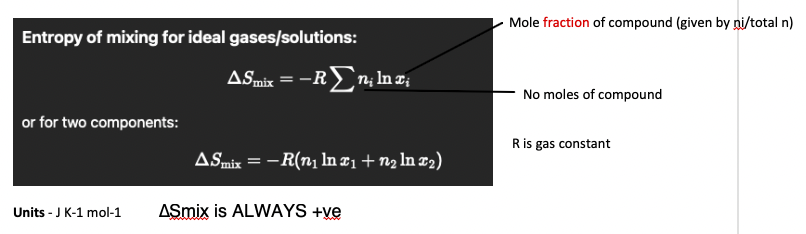
define entropy of mixing
increase in entropy that occurs when two or more substances are mixed, due to the increase in the number of possible arrangements of particles.
entropy of mixing formula
entropy of surroundings formula
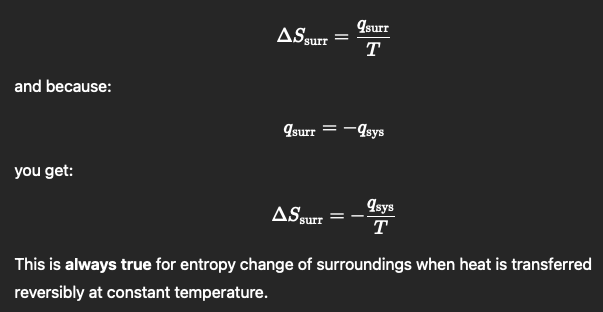
∆𝑺𝒔𝒖𝒓𝒓 = 𝒒𝒔𝒖𝒓𝒓/ 𝑻
can use regardless of whether the change in the system is reversible or not.
deriving
𝒒𝒔𝒖𝒓𝒓= −𝒒𝒔𝒚𝒔
This is from first law of TD, energy cannot be created or destroyed - only transferred. Thus:
if the system releases heat, the surroundings gain that same amount
if the system absorbs heat, the surroundings lose that amount
How to get ∆𝑺𝒔𝒖𝒓𝒓 when rev heat transfer at constant temp
How to get ∆𝑺𝒔𝒖𝒓𝒓 when constant pressure, and heat exchanged = ∆H
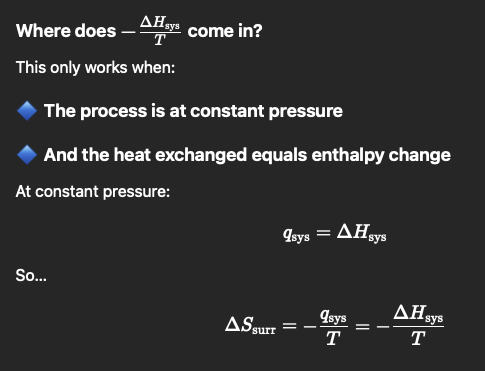
Example of sign convention with ∆𝑺tot

(this is at constant T and p)
For a reversible change (process at equilibrium): Δstot = 0, we bring in…
We make a new thing:
∆𝑮 = ∆𝑯𝐬𝐲𝐬 − 𝑻∆𝑺𝐬𝐲𝐬
also
∆𝑮 = − 𝑻∆𝑺𝐭𝐨𝐭𝐚𝐥
Also, ∆𝑮 is proportional to ∆𝑺𝐭𝐨𝐭𝐚𝐥