min 2030 final exam: Part 2 - Lec 2 - Halides Carbonates Phosphates Nesosilicates
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
halides
minerals where the anion is a halogen element
Cl, F, Br, I
larger anions than oxygen
anions have -1 charge
only 3 are common:
halite
sylvite
fluorite
halides- halite
NaCl
Face-centered cubic unit cell
Isometric
Perfect cleavage in 3 directions {100}, {010}, {001}
Very soluble in water
Tastes salty
Typically forms cubic crystals when euhedral
Sometimes “hoppered”
Moh’s 2.5
Generally colourless to white (Can even be blue, or coloured by impurities (yellow, grey, etc))

halides - sylvite
KCl
isomorphous with halite
same hardness, cleavage, crystal system and space group
commonly occurs with halite
can be colourless/white , grey, yellowish
has a strong bitter salty taste

halite and sylvite
form as evaporites- minerals precipitated from water as a result of evaporation
can be found with other evaporite minerals - sulphates and borates
can form from seawater or inland lakes
marine evaporites
forms in isolated or semi isolated basins
arid climates
deposition of chemical sed rocks- carbonates, gypsum, halite
non-marine evaporites
lakes or ponds
arid climates
deposition of chemical sed rocks- carbonates, gypsum, halite
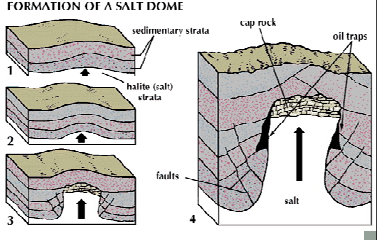
diapirs
A structure where ductile, buoyant material (like halite) pushes up through overlying sediments.
Forms salt domes.
Can act as non-porous traps for oil.
halides- fluorite
CaF2
mohs 4
isometric
octahedral cleavage
economically important
lowers melting point of metals and removes impurites
production of HF
common colours- purple, green, blue, yellow
commonly fluorescent in UV

carbonates
(CO3)2- anionic group
several are rock forming
industrial minerals, ore minerals
Calcite
Aragonite
Dolomite
Magnesite
Siderite
Rhodochrosite
Smithsonite
Malachite
Azurite
nitrates
(NO3)- anionic group
rare
borates
(BO3)- anionic group
found in evaporitic deposits
ores of boron
industrial minerals
rare
carbonates- calcite group
trigonal carbonates with 2+ cation
calcite
magnesite
siderite
rhodochrosite
smithsonite
carbonates- calcite group- calcite
CaCO3
trigonal
hardness 3
reacts vigorously with HCl
rhombohedral cleavage
habits- massive, granular, rhombohedra, scalenohedra (dog tooth)
wide range of colours- white, blue, grey, etc
rock forming mineral
accessory mineral in extremely wide range of rocks

calcite is a rock forming mineral in…
•Sedimentary carbonate rocks (limestone)
•Metamorphosed sedimentary carbonates (marble)
•Some hydrothermal veins (ore-bearing and not)
•Carbonatite magmatic rocks
•Skarn deposits
carbonatites
•Carbonate-rich plutonic and rarely volcanic
rocks
•Formed from low degree of partial melting of
upper mantle
•Calcite is abundant, may contain significant
dolomite and ankerite.
•Barite may be abundant in some deposits.
•Typically enriched in REE, Nb, Zr, Ti, P
(apatite).
• Important economic source of REE(rare earth elements) and Nb.
carbonates-calcite group- siderite
very weak HCl reaction
similar habits and cleavage to calcite
large amounts in some hydrothermal ore depsits
accessory mineral in shales, associated with coal seams
commonly brown

carbonates- dolomite group
trigonal carbonates with Ca2+
dolomite
ankerite
carbonites- dolomite group- dolomite
similar properties to calcite
commonly pinkish, colourless, grey, yellowish
rock forming mineral in
•Sedimentary carbonate rocks (dolostone)
•Metamorphosed sedimentary carbonates (dolomitic marble)
•Carbonatite magmatic rocks (sometimes)
•Skarn deposits (magnesianskarns only)
accessory mineral in a wide range of rocks
nearly impossible to discern from calcite without HCl test
but form saddle shaped crystals
dolomite is a rock forming mineral in …
•Sedimentary carbonate rocks (dolostone)
•Metamorphosed sedimentary carbonates (dolomitic marble)
•Carbonatite magmatic rocks (sometimes)
•Skarn deposits (magnesianskarns only)
carbonates - aragonite group
orthorhombic carbonates with 2+ cation
aragonite
strontianite
cerussite
carbonates- aragonite group- aragonite
CaCO3 - polymorph of calcite
orthorhombic
present in some modern sed carbonates
found in high pressure metamorphic rocks where calcite is not stable
carbonates- azurite
Cu3(CO3)2(OH)2
bright blue
light blue streak
carbonates- malachite
Cu2CO3(OH)
rich green
light green streak
Cu carbonates
azurite and malachite
can form in small quantities from the weathering of Cu-bearing minerals in the near surface (chalcopyrite), thin crusts in weathered rocks
form in large quantities in the oxide zone of supergene copper deposits- as massive, radial, or botryoidal
may occur together- especially in supergene deposits
sulphates
SO42- anionic group
Gypsum
Anhydrite
Barite
Celestine
Alunite
chromates and selenates
Cr+O and Se+O anionic groups
rare
sulphates- gypsum
CaSO4× 2H2O
monoclinic
perfect cleavage on {010} and distinct on {100} (not basal)
can have micaceous appearance
mohs 2
usually colourless, grey, yellowish
habits: massive, platy or bladed, columnar aggregates, radial aggregates, “desert rose”
occurs in larger quantities in evaporites
commonly forms near surface in weathered sed rocks
low P-T hydrothermal deposits

sulphates- anhydrite
CaSO4 - anhydrous (no water)
occurs in
evaporites with gypsum
diapirs, igneous rocks, metamorphosed evaporite rocks

how can you tell anhydrite and gypsum apart with the electron microprobe?
there would be a weight % less than 100 for gypsum- EPMA cannot detect hydrogen
anhydrite would be 100- there is no hydrogen
sulphates- barite
BaSO4
occurs in accessory to rock forming amounts in some types of hydrothermal vein deposits, MVT Pb-Zn deposits, and carbonatites
very common trace/ accessory mineral in sed carbonate rocks
common in low-T hydrothermal veins
associated with galena
industrial uses and mined economically
colours- whites, colourless, pinkish, yellowish, blue
habits- massive, granular, radial, bladed aggregates, tabular, prismatic
recognized by: habit, perfect cleavage, high density

sulphates- celestine
SrSO4 - ore of strontium
similar settings to barite
less common, but can occur in rock forming amounts in economic deposits (also in limestones)
dense - SG 4
sulphates- alunite
KAl3(SO4)2(OH)6
yellow, colourless, greyish, reddish
rare in the field
occurs in veins and zones of alteration in K-rich volcanic rocks
phosphates
(PO4)3- anionic groups
some are very common as accessory minerals in a wide range of environments
present in larger, even rock forming amounts in some unusual environments
Apatite
Monazite
Turquoise
Pyromorphite
Vivianite
phosphates- apatite
three Ca phosphates which form a solid solution series based on different -1 charged anions
fluorapatite- most common
hydroxylapatite- very uncommon (bones)
chlorapatite- very rare
phosphates- apatite
Ca5(PO4)3F (fluora-)
prismatic, euhedral or subhedral crystals
hexagonal
poor cleavage
mohs 5
typically green-blue, grey, colourless, reddish brown
very common trace/accessory mineral
rarely occurs in rock forming amounts in coarse grained carbonate intrusive rocks
mined for use in fertilizer
useful for fission-track dating
fission track dating
•Uranium radioactive decay ejects
particles which cause ‘tracks’ of
damage in a mineral grain
•They can be used for
thermochronology–determining
the age at which a rock cooled
below the annealing temperature
of apatite or other minerals
phosphates- monazite
three monazite species which forma solid solution based on the dominant rare earth element
(REE)PO4
Ce- most common
Nd- neodymium
La- Lanthanum
common trace mineral, rare accessory mineral (except in REE-rich deposits)
can be age dated using the U-Pb system
ore mineral of REE and Th
most abundant in carbonatites, alkaline plutonic rocks, and some type of granitic pegmatite

monazite thin section
olive-yellow green
high relief
high birefringence
biaxial- monoclinic
commonly has radio halos
arsenates- annabergite
Ni3(AsO4)2 × 8H2O
green, low P-T alteration of Ni-As bearing ores
in tailings cobalt, Ontario 5 element veins
arsenates- erythrite
Co3(AsO4)2 × 8H2O
“cobalt bloom”
bright pink, low P-T, near-surface alteration of Co-As-bearing ores
in tailings at cobalt, ontario 5 elements veins
silicates
silica tetrahedron forms the basis of this mineral class
(SiO4)4-
most abundant mineral group in earths crust
divided into subclasses based on the structural arrangement of silica tetrahedra
composition of most rocks
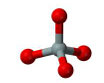
nesosilicates
single isolated silica tetrahedra (SiO44-)
Olivine
Garnet
Zircon
Kyanite
Andalusite
Sillimanite
Topaz
Staurolite
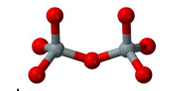
sorosilicates
double silica tetrahedra joined at one vertex (Si2O76-)
Epidote
Zoisite
Lawsonite
Vesuvianite (Idocrase)
Hemimorphite
Allanite
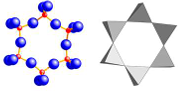
cyclosilicates
Silica tetrahedra arranged into rings. Number of tetrahedra is variable in different mineral species. (4, 6 and 8)
Beryl
Tourmaline
Cordierite
Benitoite
Dioptase
inosilicates
silica tetrahedra form single or double chains
Single-chain (pyroxenes):
Augite
Diopside
Enstatite
Hedenbergite
Double-chain (amphiboles):
Hornblende
Actinolite
Tremolite
Glaucophane

phyllosilicates
silica tetrahedra form sheets
Muscovite
Biotite
Chlorite
Kaolinite
Illite
Smectite (Montmorillonite)
Serpentine
Talc
tectosilicates
silica tetrahedra form 3 dimensional framework structures
Quartz
Orthoclase (K-feldspar)
Plagioclase feldspar
Microcline
Albite
Nepheline
Leucite
Sodalite

nesosilicates- olivine
group: M2SiO4
M= Ca, Fe, Mn, Ni, Mg
forsterite-fayalite solid solution series most common
orthorhombic
mohs 7
no cleavage, conchoidal fracture
typically yellow-green, green, brown
common in ultramafic and mafic rocks, often rock forming amounts
extremely abundant in the mantle
can occur in Mg-rich marbles metamorphosed at high grade
does not occur with quartz!
weathers very easily at the surface
nickel laterites
Ni ore deposits formed from the intense weathering of Ni-bearing olivine in ultramafic rocks
nesosilicates- garnet group
generalized formula: X3Z2(SiO4)3
isometric -equant, dodecahedral
mohs 6.5-7.5
no cleavage
typically adamantine or glassy
wide range of colours- depends on concentration of end-members, Cr has strong green colouring effect
The most common end-members are:
Grossular: Ca₃Al₂(SiO₄)₃
Almandine: Fe²⁺₃Al₂(SiO₄)₃
Pyrope: Mg₃Al₂(SiO₄)₃
Spessartine: Mn²⁺₃Al₂(SiO₄)₃
Andradite: Ca₃Fe³⁺₂(SiO₄)₃
Uvarovite: Ca₃Cr₂(SiO₄)₃ (rare, only in Cr-rich rocks)
spessartine
orange to red garnet
occur in granites and granite pegmatites

pyrope, almandine, andradite
red to reddish brown garnet

grossular
colourless, pinkish to orange garnet
common rock types that garnets occur in
Grossular- skarns and contact-metamorphosed marbles/limestones, rodingites
Almandine- Metamorphic rocks (gneiss, schist), granites
Pyrope- Kimberlites (xenocrysts), Mg-rich high pressure metamorphic rocks
Spessartine- Granites and granitic pegmatites
Andradite- skarn deposits, serpentinites
Urarovite- altered chromite deposits