Transport of Oxygen, Carbon Dioxide, and Hydrogen Ions in Blood
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
1L of arterial blood contains (oxygen)
dissolved = 3 mL
bound to haemogllobin = 197
total = 200 mL
cardiac output
5L/ min
O2 carried to tissues in 1 min
5L/min x 200 ml = 1000 mL
haemoglobin structure
2 alpha chains, 2 beta chains
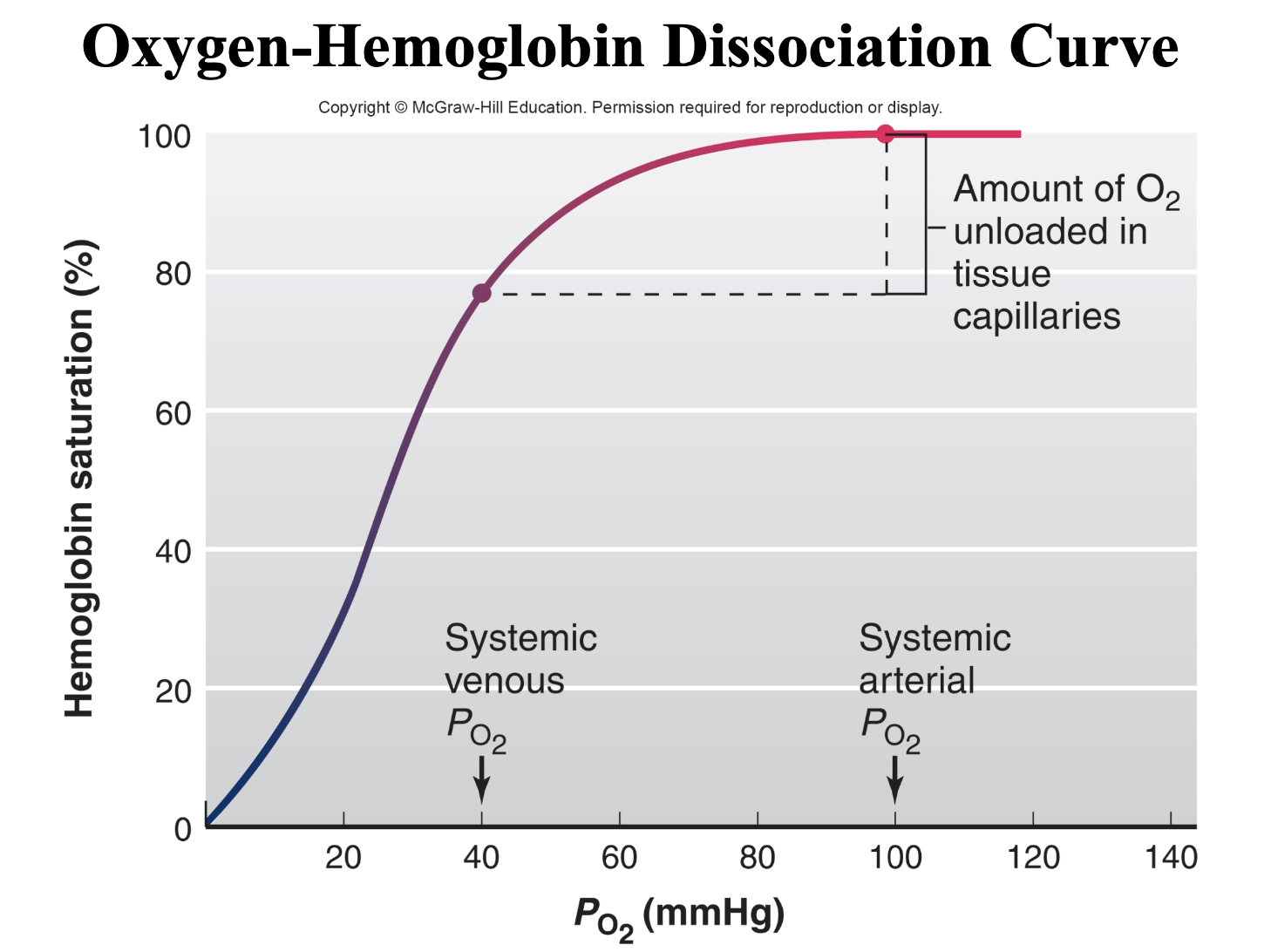
normal systemic arterial PO2
100 mmHg
normal systemic mixed venous PO2
40 mmHg
The major determinant of the degree to which hemoglobin is saturated with oxygen is
blood PO2.
-normal systemic arterial PO2 of 100 mmHg= 100% saturated hemoglobin
- normal systemic mixed venous PO2 of 40 mmHg = Hemoglobin is 75% saturated
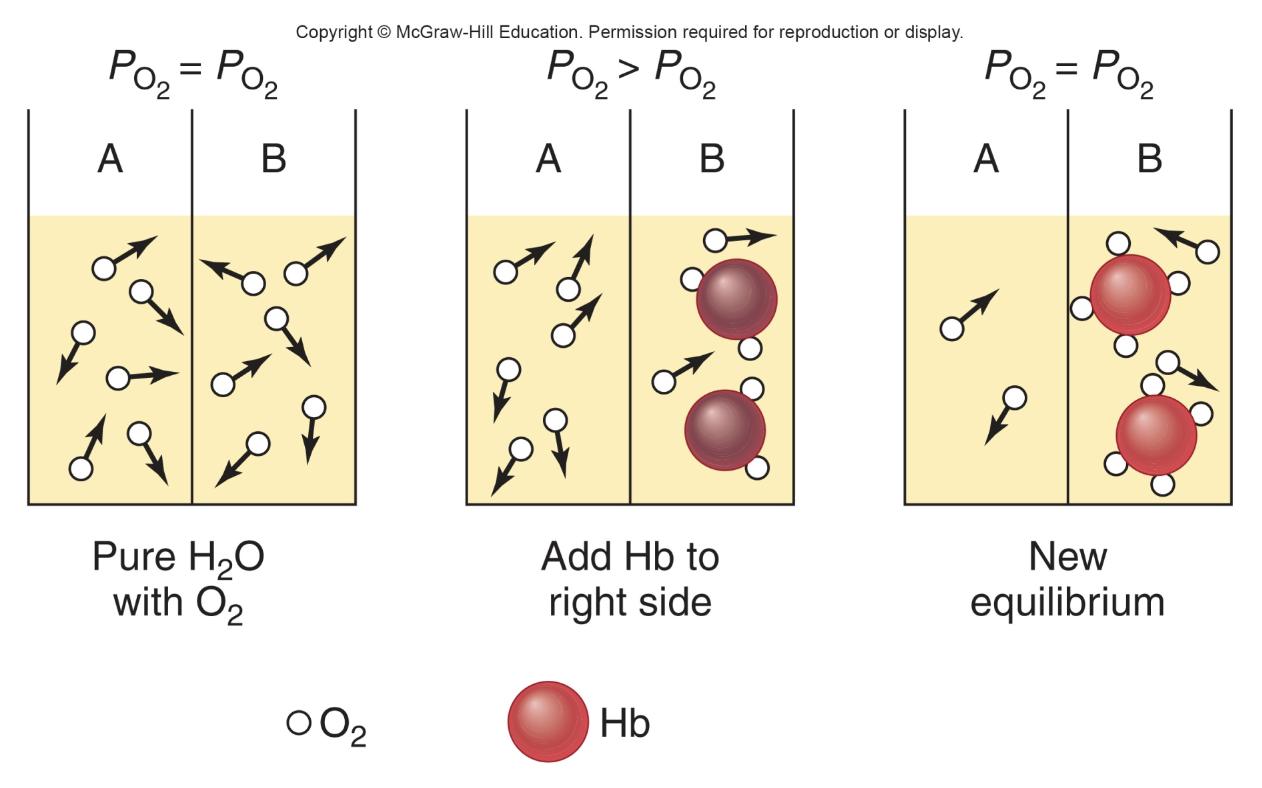
Effect of Hemoglobin Binding on Oxygen conc gradient / equilibrium
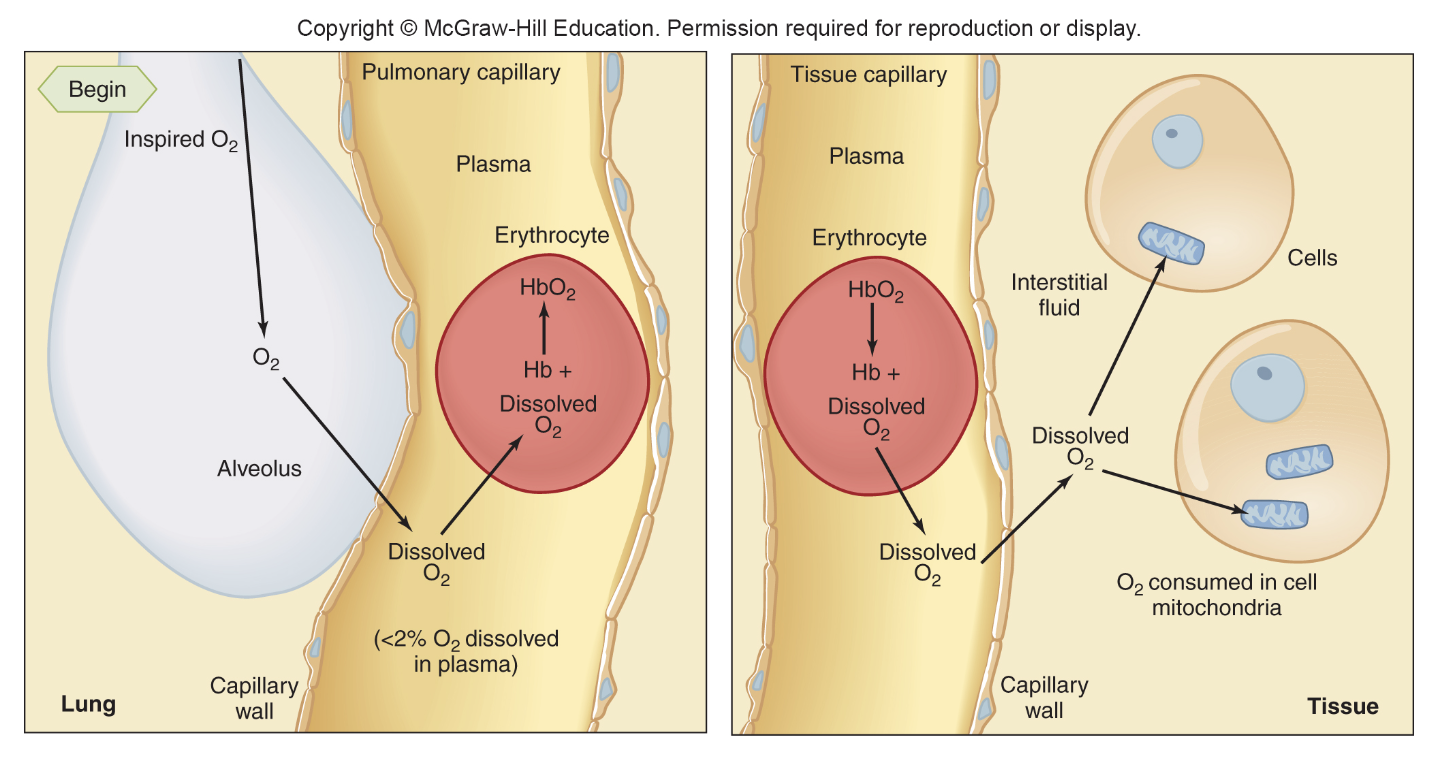
loading and unloading of O2
Carbon Monoxide Poisoning
• It is the leading cause of death from fire.
• CO is an odorless, colorless gas that competes with O2 for the binding sites on the hemoglobin.
• It has a 200-times greater affinity for hemoglobin than O2 does.
CO effect on oxygen–hemoglobin dissociation curve of the remaining oxyhemoglobin
-shift to the left (increases affinity to oxygen)
-decreasing the unloading of oxygen from hemoglobin in the tissues.
symptoms of CO poisoning
confusion, respiratory distress, the skin becomes cherry red. NO CYANOSIS is detectable.
treatment of CO poisoning
hyperbaric treatment (breathing 100% oxygen in a pressurized chamber) or 100% oxygen is used.
At any given PO2, what other factors influence the degree of hemoglobin saturation.
– PCO2,
– H+ concentration,
– temperature,
– the concentration of a substance produced by erythrocytes called 2,3-diphosphoglycerate (DPG)
– and the presence of fetal hemoglobin.
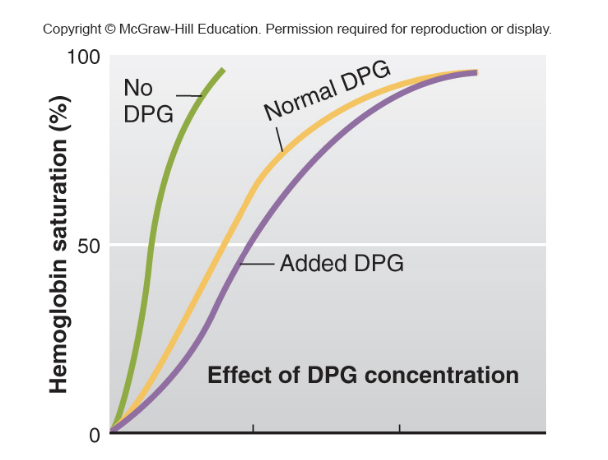
DPG effect
more DPG = less affinity
less DPG = more affinity
2,3-DPG binds to hemoglobin, reducing its affinity for oxygen. This promotes the release of oxygen from hemoglobin into tissues that need it.
In the lungs, oxygen binds to hemoglobin (forming oxyhemoglobin) with a high affinity.
In the tissues, where oxygen is needed, 2,3-DPG binds to hemoglobin, causing a conformational change that lowers hemoglobin's affinity for oxygen, allowing oxygen to be released and delivered to the tissues.
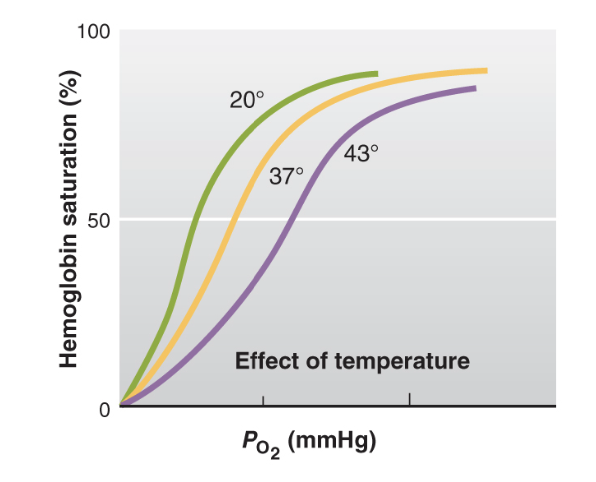
temp effect on affinity
-When the temperature increases, such as during exercise, fever, or in warmer environments, the affinity of hemoglobin for oxygen decreases.
-In extreme cold, the lower temperature can increase hemoglobin's affinity for oxygen, which could reduce oxygen delivery to tissues, potentially causing hypoxia (insufficient oxygen) in the extremities.
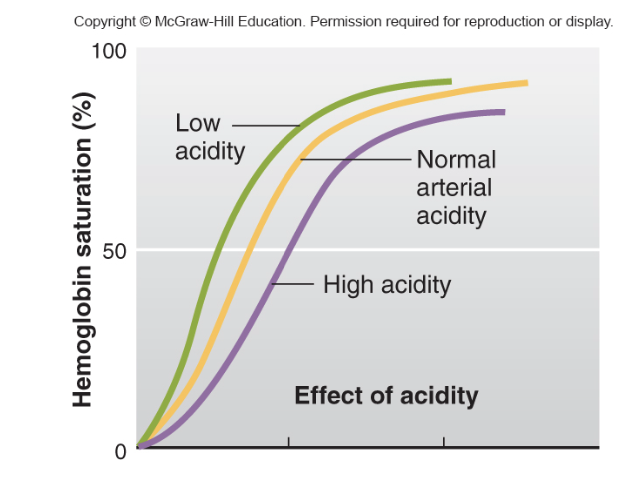
pH effect
-When the pH decreases (i.e., the environment becomes more acidic), hemoglobin's affinity for oxygen decreases.
Exercise —> lactic acid —> low pH —> need more O2 to muscles
-When the pH increases (i.e., the environment becomes more alkaline), hemoglobin’s affinity for oxygen increases.
lung pH is alkaline to increase affinity
Acidic pH (low pH): Decreases hemoglobin's affinity for oxygen, promoting oxygen release in metabolically active tissues.
Alkaline pH (high pH): Increases hemoglobin's affinity for oxygen, aiding oxygen binding in the lungs.
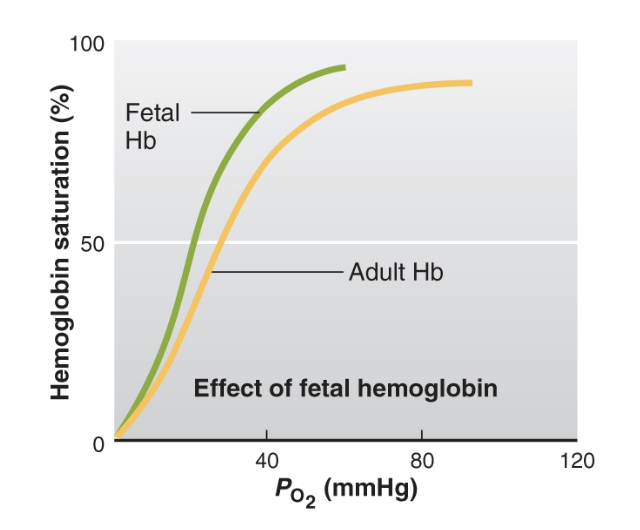
fetal Hb
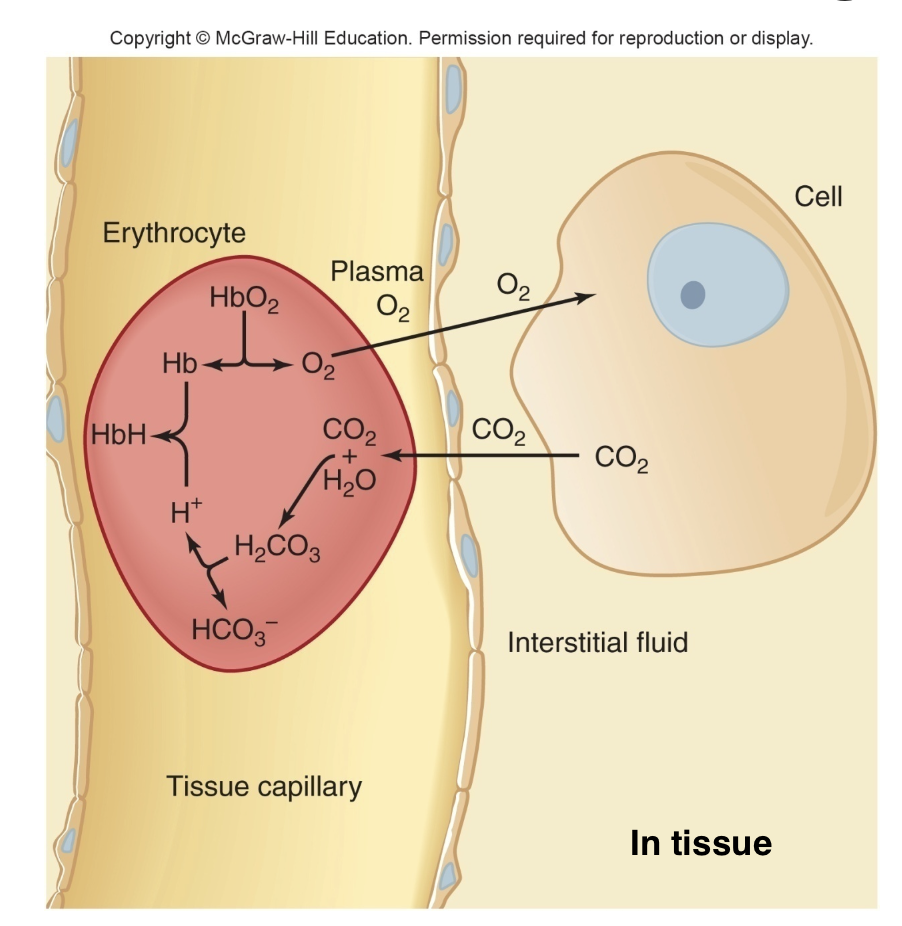
Methods of Transport of Carbon Dioxide in Blood
– 10% remain dissolved in plasma and erythrocytes
– 25% to 30% combine in the erythrocytes with deoxyhemoglobin to form carbaminohemoglobin
– 60% to 65% combine in the erythrocytes with water to form carbonic acid, which then dissociates to yield HCO3− and H+
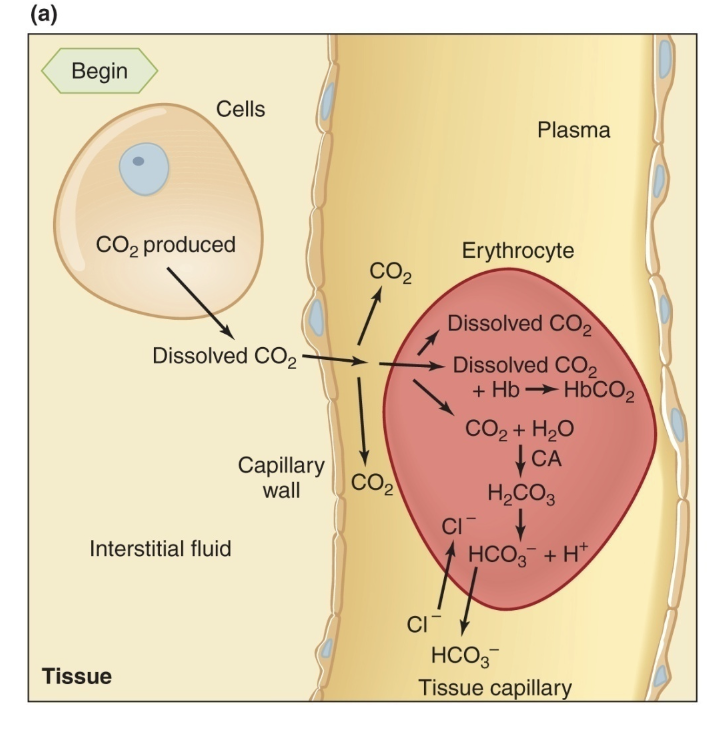
unloading of CO2
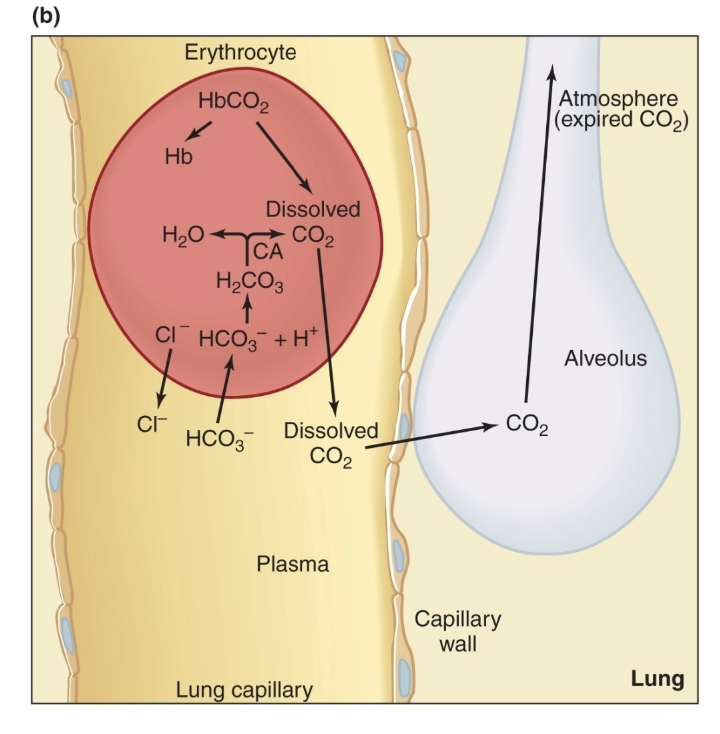
Transport of Hydrogen Ions Between Tissues and Lungs
in tissues: CO2 moves in converted to bicarbonate and H+, H+ binds to Hb
in lungs: O2 displaced H+ from Hb, H+ binds to bicarbonate —> carbonic acid —> CO2 and H2O, CO2 diffuses out
Respiratory acidosis
increase in the acidity of the blood due to increased CO2, which occurs, for example, during hypoventilation.
Respiratory alkalosis
a decrease in the acidity of the blood due to decreased CO2, which occurs, for example, during hyperventilation.
Causes of respiratory acidosis
Lung disease, depression of the respiratory center by drugs or disease, nerve or muscle disorders, or even holding one’s breath, COPD.
Compensations for respiratory acidosis
Organ: Kidneys
Response: The kidneys try to increase H⁺ excretion and reabsorb more HCO₃⁻ (bicarbonate).
Effect: This raises blood pH back toward normal.
Causes of respiratory alkalosis
– Fever, anxiety, and aspirin poisoning
Compensations for respiratory alkalosis
– Chemical buffer systems liberate H+ to diminish the severity of the alkalosis
-Organ: Kidneys
Response: The kidneys try to retain H⁺ and excrete more HCO₃⁻
Effect: This lowers blood pH back toward normal
what happens to ventilation, CO2 removal and H+ generation from CO2 in nonrespiratory/metabolic acidosis
more ventilation, more CO2 removal, so less H+ generation
what happens to ventilation, CO2 removal and H+ generation from CO2 in nonrespiratory/metabolic alkalosis
less ventilation, less CO2 removal, more H+ generation
CO2 effect on O2 affinity to Hb
increased CO2 = decreased affinity
hemoglobin affinity for H+ and CO2 is decreased by
increased PO2