Gases in the atmosphere
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What are the 4 most abundant gases in the atmosphere?
nitrogen → 78%
oxygen → 21%
argon → 0.9%
carbon dioxide → 0.04%
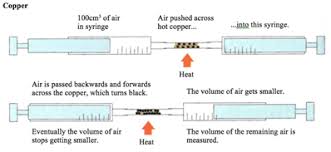
Describe an experiment to calculate the % of oxygen in the air
1. a pile of copper powder is placed in a glass tube connected to 2 gas syringes with one end containing 100cm3 of air and the other containing 0cm3
2. the joints on the apparatus are checked & sealed to avoid any other air from entering the experiment
3. the copper is heated with a Bunsen burner & the gas syringes slowly passes air to one another
4. the copper turns black as it has reacted with the oxygen to form copper II oxide
5. the % of oxygen in the air can be calculated by subtracting the intial volume of air (100cm3) by the final volume of air
What is combustion?
- a reaction where a substance burns in oxygen
- the substance gains oxygen so it is an oxidation
What happens in the combustion of magnesium, hydrogen & sulfur?
MAGNESIUM → 2Mg + O2 → 2MgO
HYDROGEN → 2H2 + O2 → 2H2O
SULFUR → S + O2 → SO2
What is thermal decomposition?
a reaction where a substance is broken down using heat energy
What happens when a metal carbonate undergoes thermal decomposition?
metal carbonate → metal oxide + carbon dioxide
(e.g. CuCO3 → CuO + CO2)
What are some greenhouse gases?
- carbon dioxide (CO2)
- methane (CH2)
- water vapour
What is the greenhouse gas effect?
When energy from the sun travels to the Earth as short wavelength radiation, the surface of the Earth then radiates the energy as long wavelength radiation.
Some of the long wavelength radiation interacts with the greenhouse gases in the atmosphere
Because the energy is trapped in the atmosphere, this causes the temperature of the atmosphere to increase
Leading to global warming