B1.1 CARBOHYDRATES AND LIPIDS
1/23
Earn XP
Description and Tags
help me help me help me help me he lp me
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
explain the chemical properties of a carbon atom which allows for the formation of diverse compounds upon which life is based
is an organic compound (compound containing C and found in living things) (except for carbonates, CO2, cyanide)
carbon can form up to four covalent bonds (single or double) with other carbons or non-metallic elements (O,H,S,N,P)
structures can vary from unbranched, branched, ring, cyclic
bonds between carbon is extremely stable
more than most atoms in the periodic table, results in formation of diverse compounds of which life is based on
EXAMPLE: amino acids, nucleotides, carbs
outline the production of macromolecules by condensation reaction that link monomers to form a polymer
monomers are recurring subunits covalently joined to form polymers
polymers form monomers by condensation reactions
water molecule removed as a byproduct
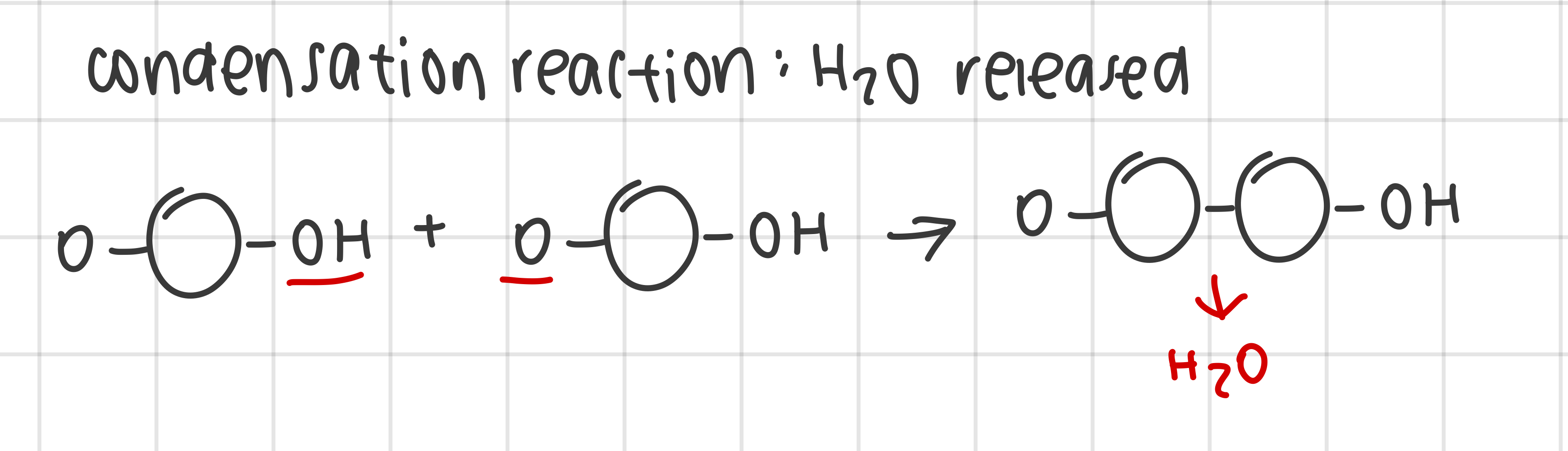
outline the the digestion of polymers into monomers by hydrolysis reactions
polymers broken down into monomers by hydrolysis
water molecule added as a reactant to facilitate the breakage of covalent bonds between the monomers
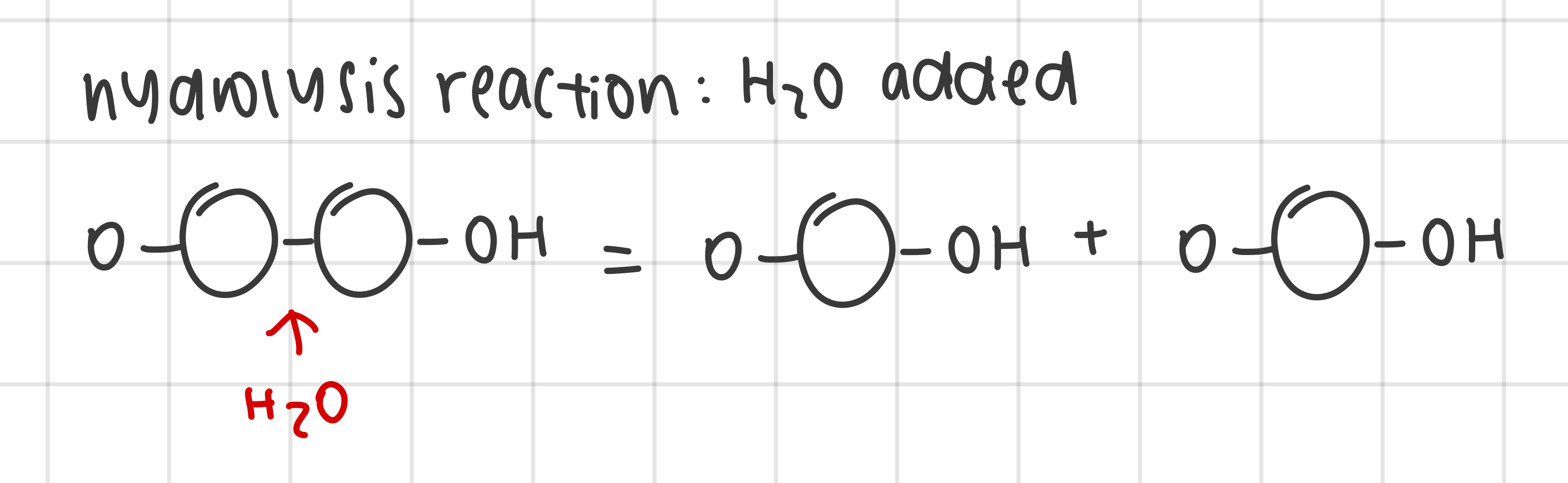
carbohydrates, proteins and nucleic acids
examples: (subcatagory) monomers → (catagory) polymers (bond between monomers)
examples: monomers → polymers (bond between monomers)
monosaccharide, disaccharide, polysaccharide → carbohydrate (glycosidic bond)
amino acid, polypeptide → protein (peptide bond)
nucleotide, polynucleotide → nucleic acids (phosphodiester bond)
outline the form and function of monosaccharides
carbohydrates composed of reaccuring monomers called monosaccharides
they are single sugar units
serve as source of energy for cells, oxidised to produce large quantities of ATP
solubility: polar molecules so they dissolve in H2O
stability: cyclic/ring structure
transport: soluble and stable, good transport in aqueous solutions
potential energy: have high energy due to presence of multiple C–H bonds
classified by the number of carbon atoms
pentose(5C) - deoxyribose, ribose
hexose(6C) - primarily used as energy source for cellular respiration
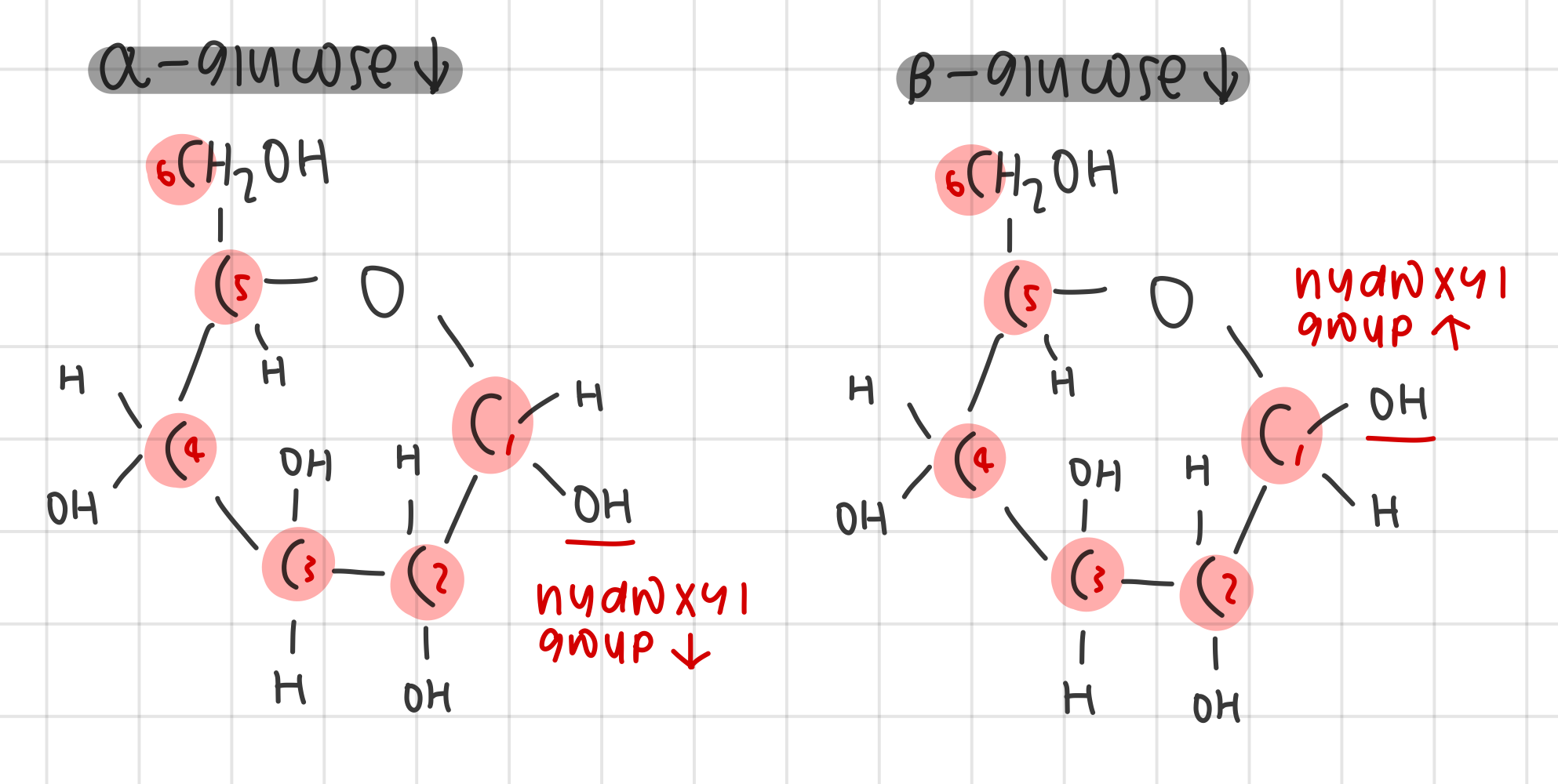
state the 2 isomers of glucose and draw them
2 isomers: α-glucose and β-glucose
α-glucose - OH group point down on 1’ C
β-glucose - OH group point up on 1’ C
use glucose as an example of a monosaccharide to outline the structure, properties, and function
isomers play an important role in the formation of different structures of polysaccharides
soluble molecule
presence of many -OH groups cause it to be polar
O in the ring is slightly negative
formation of hydrogen bonds between hydroxyl group and water causes it to dissolve easily and can be transported in blood and in fluids between cells
stable molecule
cyclic structure
polysaccharides it forms are also stable
glycosidic bonds within glucose are stable covalent bonds
yields a lot of chemical energy when the covalent bonds are hydrolysed
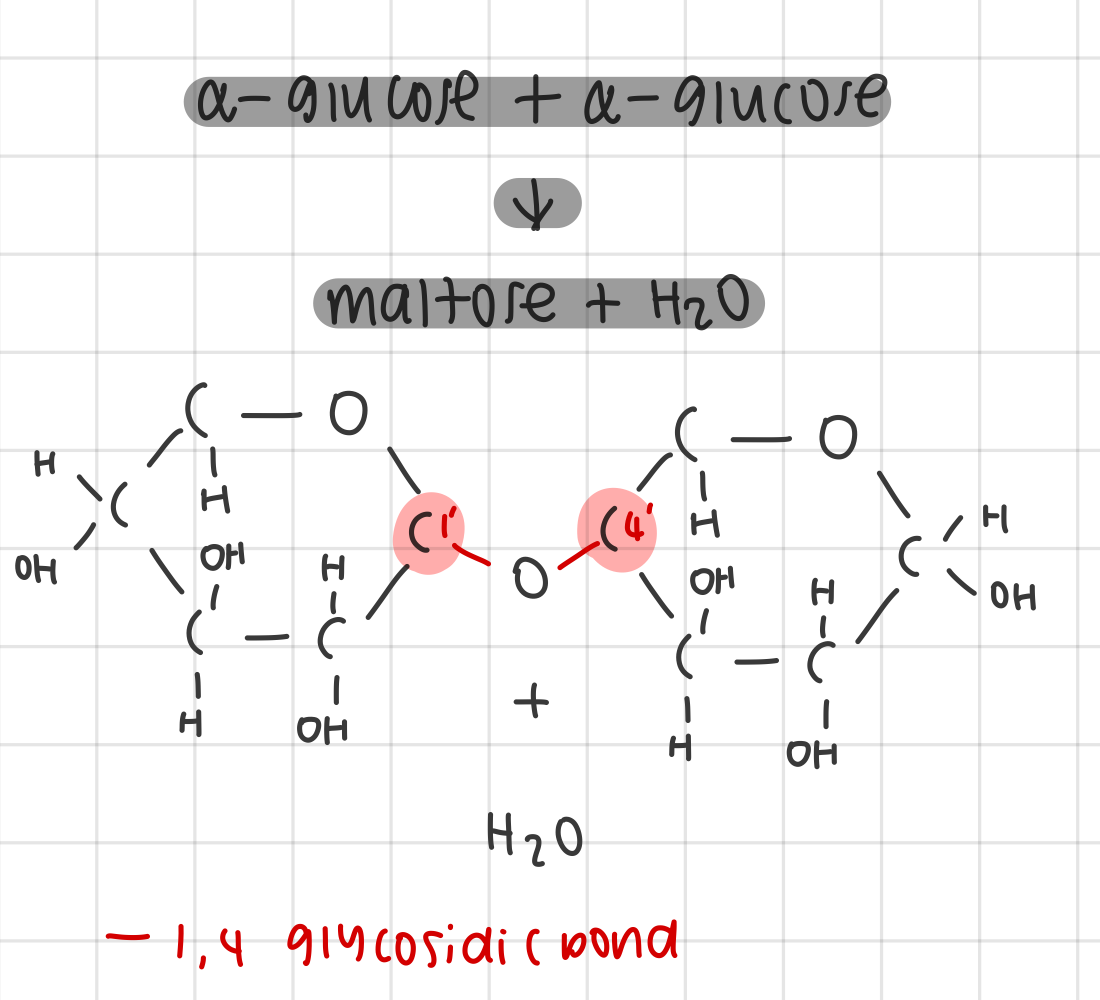
outline the formation of the disaccharide, maltose
condensation reaction between 2 α-glucose creates maltose + H2O
1,4 glycosidic bond is formed between the groups attached to carbon-1 of the first glucose molecule and carbon-4 or the second
explain how polysaccharides can act as energy storage compounds
energy storage compounds
easy to add/remove α-glucose monomers to build/imobilise energy stores
insoluble due to large molecular size
examples: starch in plants, glycogen in animals (both are polymers made up of α-glucose)
structural compounds
branching to adopt more compact structures (however large sizes are insoluble in water, therefore they cannot be transported within aqueous solutions such as blood)
examples: cellulose (made up of β-glucose)
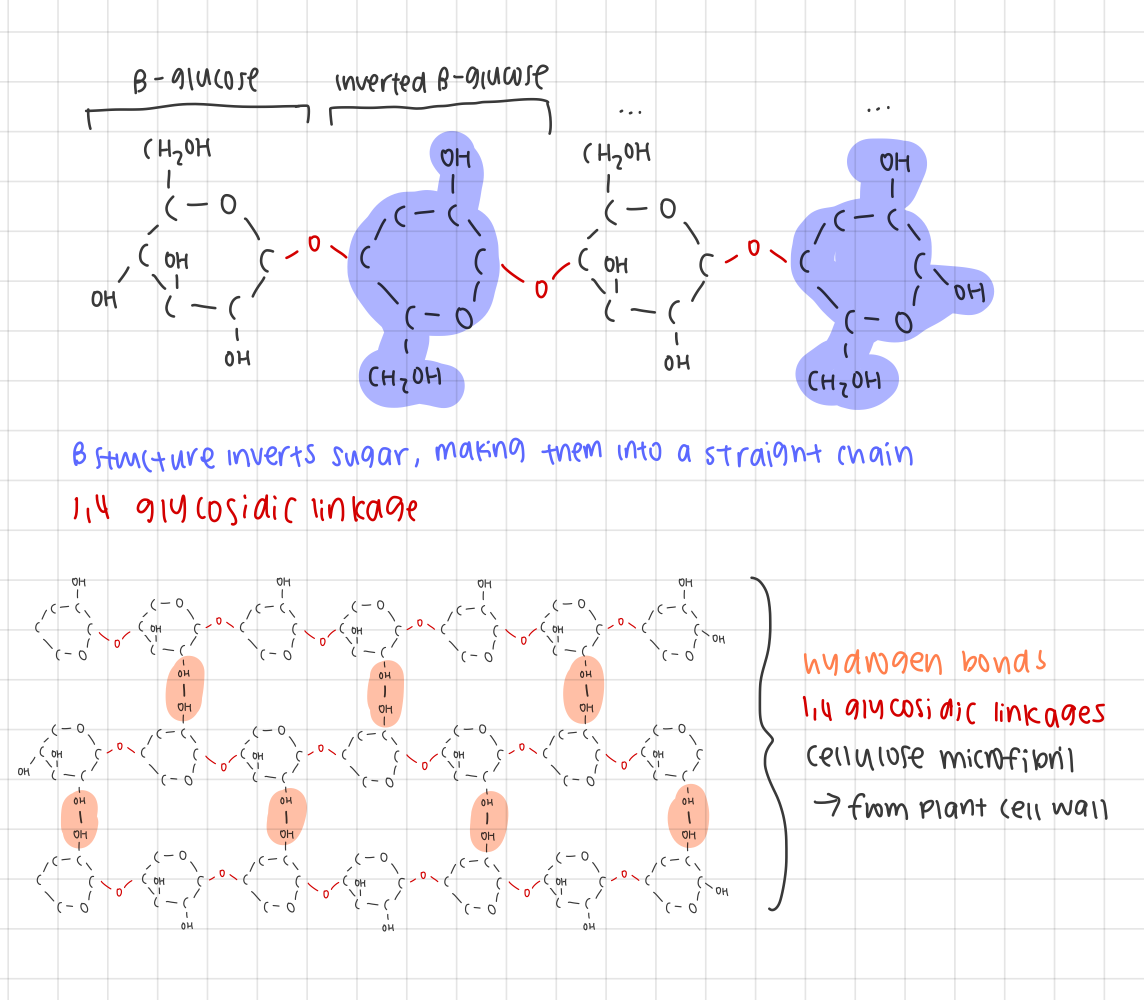
examples of polysaccharides: outline the structure of cellulose and how it supports the function
composed of β-glucose subunits with β-1,4 glycosidic bonds
alternating orientation of β-glucose monomers to create a straight chain
straight chain is then grouped in bundles and cross-linked with hydrogen bonds
increases structural identity
prevents access to water, making cellulose resistant to hydrolysis (acts as an excellent structural compound)
structure:
composed of β-glucose subunits with β-1,4 glycosidic bonds
alternating orientation of β-glucose monomers to create a straight chain
straight chain is then grouped in bundles and cross-linked with hydrogen bonds
function:
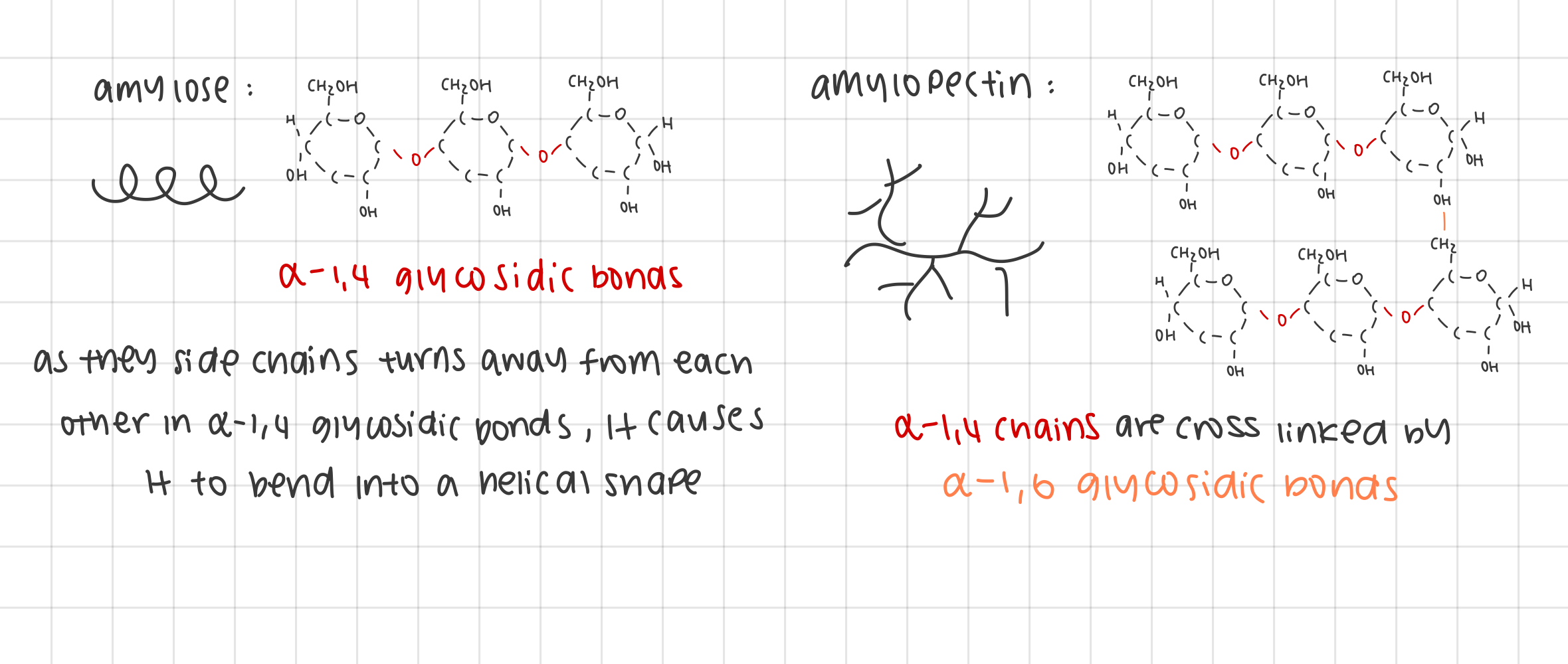
examples of polysaccharides: outline the structure of starch and how it supports the function
structure:
composed of 2 α-glucose subunits, amylose and amylopectin
amylose: 1,4 glycosidic linkages formed into helical arrangement
amylopectin: 1,4 glycosidic and 1,6 glycosidic linkages
Insoluble due to large molecular size
function:
storage molecule in plants
insoluble in H2O
will not affect the osmotic potential
compact structure
able to store alot of glucose in a very small space
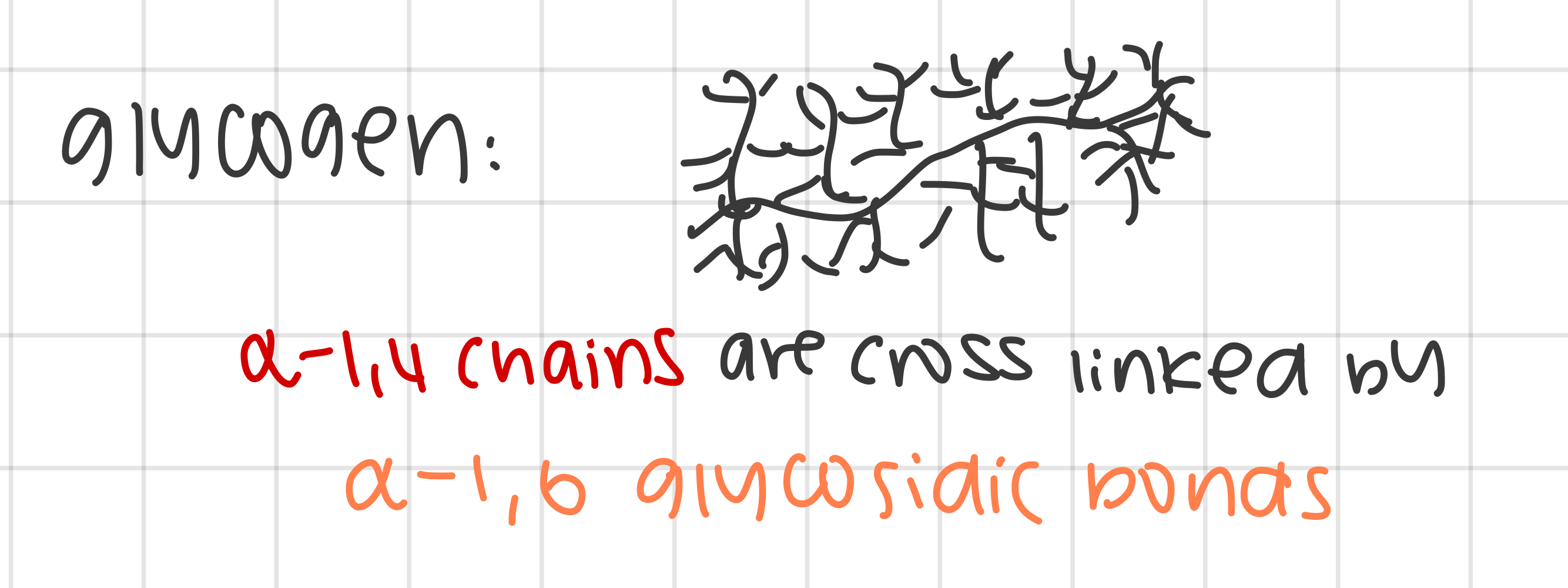
examples of polysaccharides: outline the structure of glycogen and how it supports the function
structure:
composed of α-glucose (similar to amylopectin but more cross-links, highly branched and shorter α 1,4 chains)
function:
storage molecule in animals
insoluble in H2O
will not affect the osmotic potential
compact structure
able to store alot of glucose in a very small space
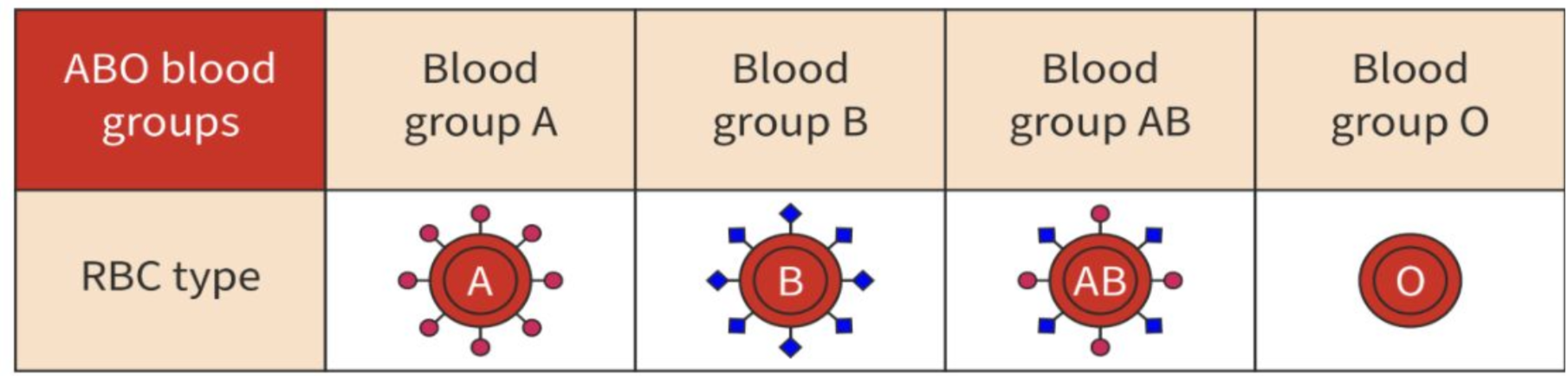
explain the role of glycoproteins in cell–cell recognition
glycoproteins are proteins that are attached to carbohydrates to perform cell-cell recognition, acting as markers on the cell surface
example: human RBC (ABO blood types)
categorised into blood types by surface glycoproteins which function as identification tags for the immune system
outline the properties of lipids and give examples of the different types
lipids are hydrophobic
non-polar organic molecules
composed of hydrocarbon chains/rings (non-polar covalent bonds)
they are fatty acids
soluble in non-polar solvents and slightly soluble in polar solvents
examples of lipids include waxes, steroids, phospholipids, triglycerides
outline what waxes are, its structure and its function
composed of C, H , O atoms
hydrophobic
contists of an ester
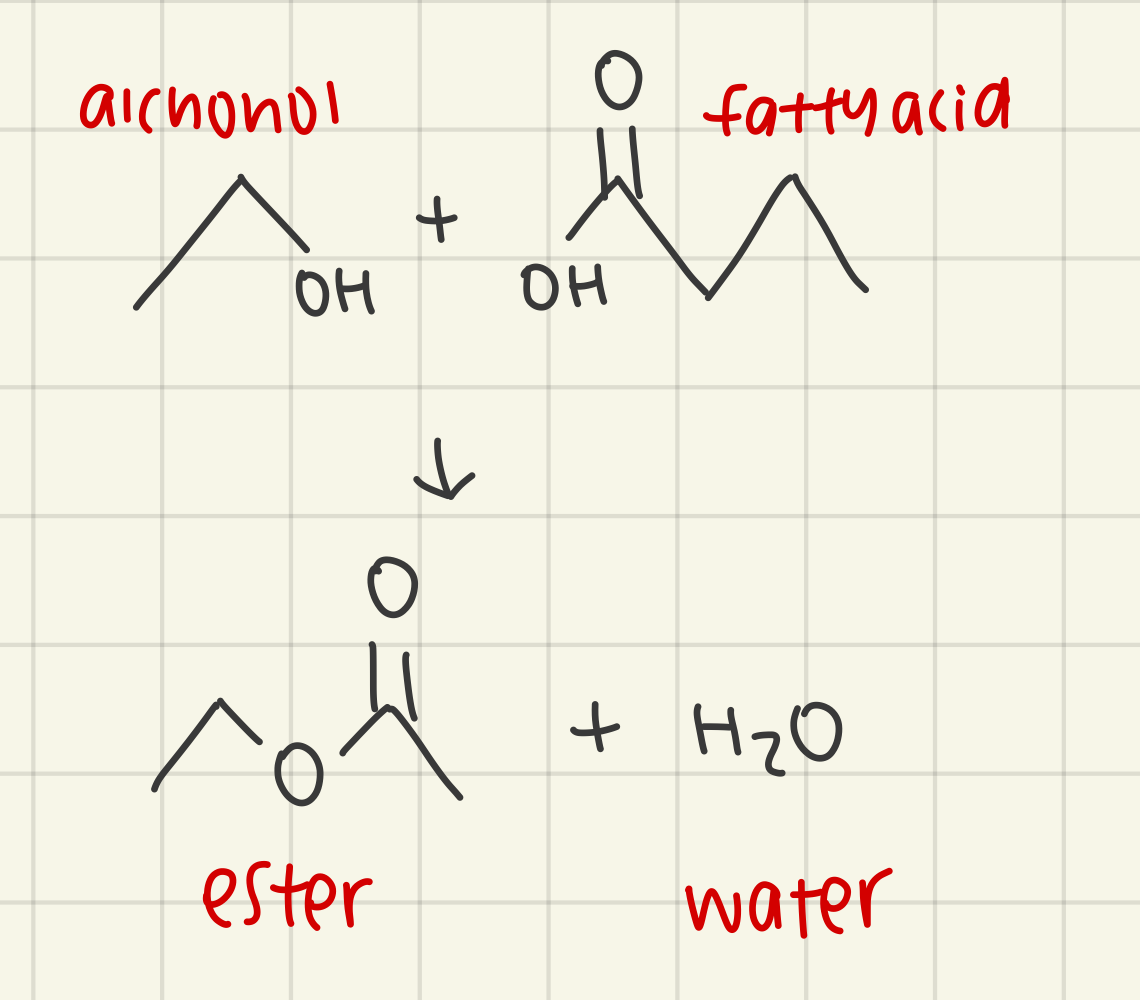
a fatty acid and alchohol undergoes a condensation reaction to make wax, h2o is released and the fatty acid and alcohol are linked by an ester bond
outline what steroids are, its structure and its function
composed of C, H, O atoms
made of 4 hydrocarbon rings fused together
hydrophobic
used as building blocks for steroid hormone (testosterone, progesterone)
outline what triglycerides are, its structure and its function
composed of C, H, O atoms
don’t release that much energy when oxidised
hydrophobic
helps with long term energy storage as it will not affect the osmotic pressure
made up of glycerol and 3 fatty acid molecules (cis, trans, saturated
outline what phospholipids are, its structure and its function
composed of C, H, O, P molecules
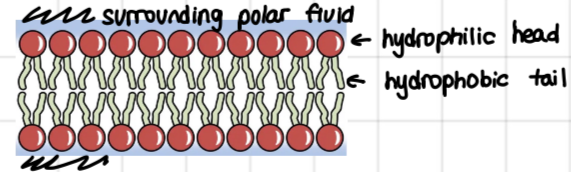
consists of a polar head which is hydrophilic and 2 non polar tails which are hydrophobic (it is amphipathic)
phospholipids spontaneously arrange into bilayer which is held by hydrophobic interactions between non polar tails
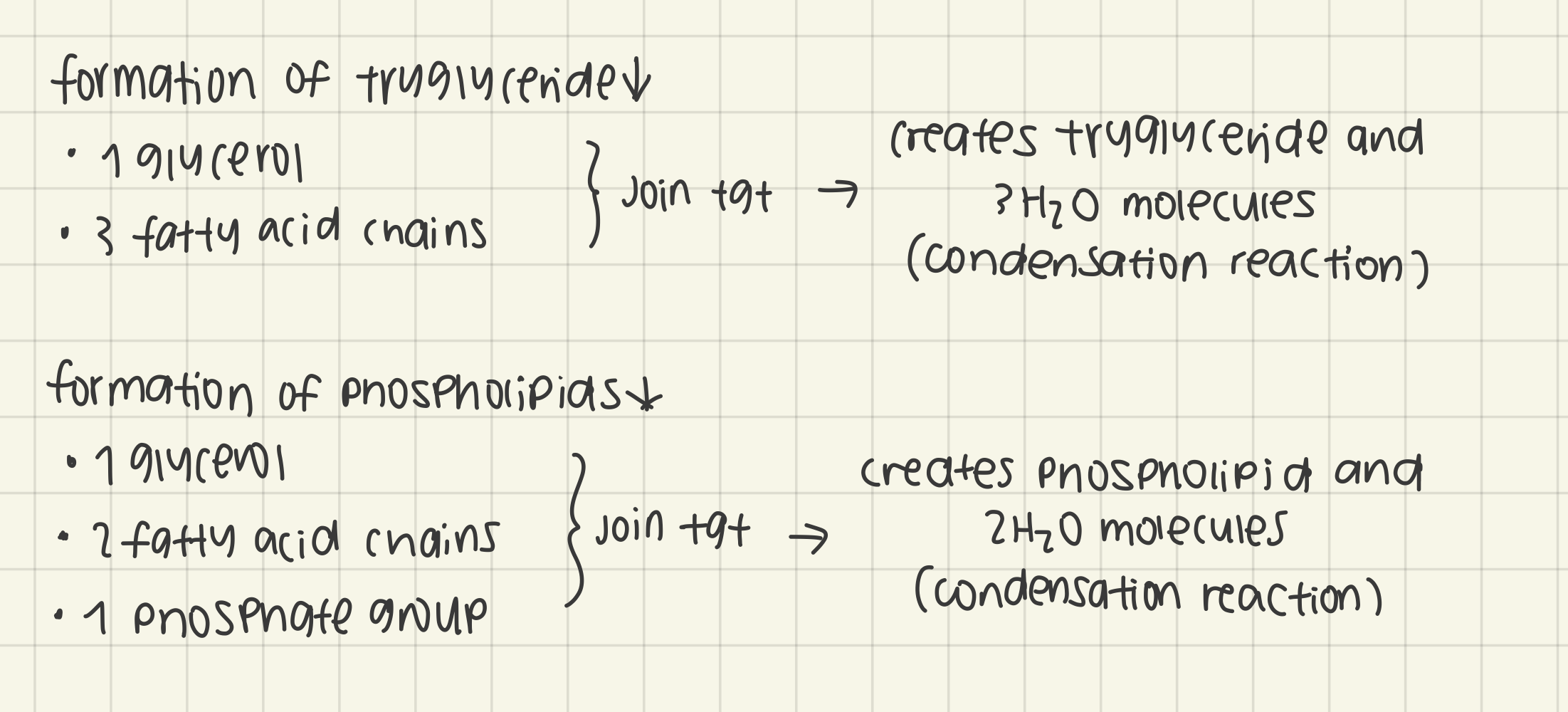
outline the formation of triglycerides and phospholipids by condensation reactions
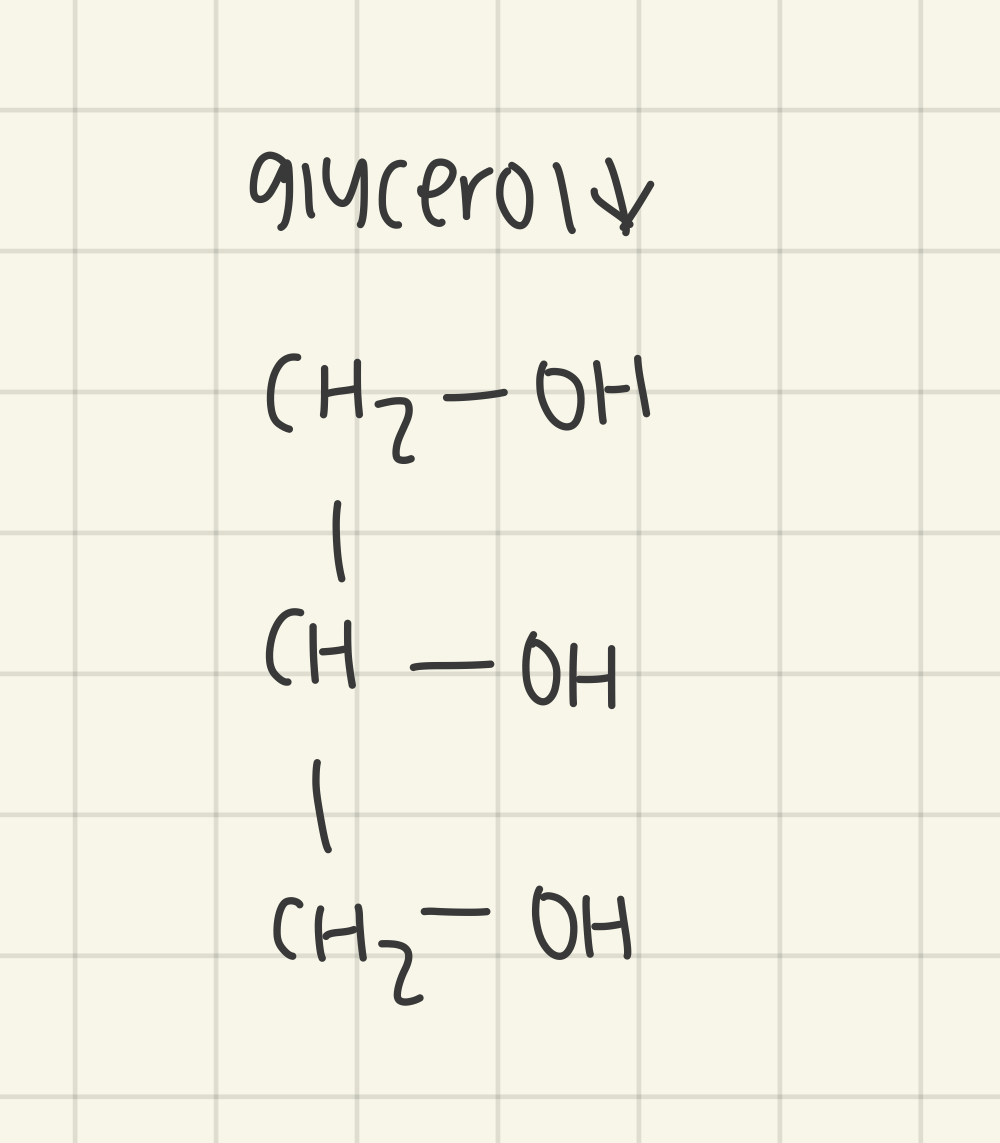
outline the structure and properties of glycerol
highly soluble
outline the different types of fatty acids, the structure, and its properties
mono-unsaturated: one double bond
poly-unsaturated: more than one double bond

explain the use of saturated and unsaturated fatty acids in oils and fats used for energy storage in plants and endotherms respectively

explain why triglycerides in adipose tissues are ideal for energy storage and thermal insulation

explain the ability of non-polar steroids to pass through the phospholipid bilayer
steroids are composed of four fused C rings
they are non polar meaning they are hydrophobic
they are small in size, allowing them to fit in between the bilayer
since the phospholipid bilayer consists of hydrophobic tails, the steroids are able to pass through as they are the same medium steroid hormone freely pass through the phospholipid bilayer and bind to receptors within cell, but cannot be transported by blood
steroid hormone freely pass through the phospholipid bilayer and bind to receptors within cell, but cannot be transported by blood