10.4 - The Haber Process & Use of NPK Fertilizers
1/4
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
Outline the key points of the Haber process. Include the use of the product.
The purified H2 and N2 gases are passed over an iron (Fe) catalyst at a high temperature (about 450°C) and a high pressure (about 200 atm).
Iron speeds up the rate of reaction, so a lower temperature could be used in the process.
Some of the hydrogen and nitrogen reacts to form ammonia (N2 + 3H2 ⇌ 2NH3)
This reaction is reversible so ammonia breaks down again into nitrogen and hydrogen.
On cooling, the ammonia liquefies and is removed. The remaining hydrogen and nitrogen are recycled. This means almost no material is wasted.
Ammonia is used for production of nitrogen-containing fertilizers.
Explain why the Haber process uses high temperature and pressure conditions and why this is a compromise.
Compromise between rate and yield:
The reaction is exothermic. An optimum temperature of 450°C is used. Using a lower temperature would give a higher yield, but the rate of NH3 production would be too slow.
A pressure of 200 atm is used. Using a higher pressure would give a higher yield but would be too expensive, because of the cost of energy to produce the high pressure.
How are compounds of nitrogen, phosphorus, and potassium used?
Compounds of nitrogen, phosphorus, and potassium are used as fertilizers to improve agricultural productivity. NPK fertilizers contain compounds of all three elements.
How is industrial production of NPK fertilizers achieved?
Ammonia can be used to manufacture ammonium salts. The ammonium sulfate, phosphate, and nitrate can be produced by reaction of ammonia with the requisite acid.
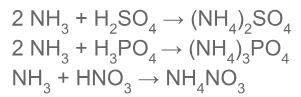
How is the phosphate rock utilized in the production of fertilizers?
Phosphate rock is reacted with nitric acid to produce phosphoric acid and calcium nitrate.
Phosphate rock can be reacted with sulfuric acid to produce a mixture of calcium phosphate and calcium sulfate.
Phosphate rock can be reacted with phosphoric acid to produce calcium phosphate.