Ch. 5 In-Class Notes--Reactions of Alkenes and Alkynes
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
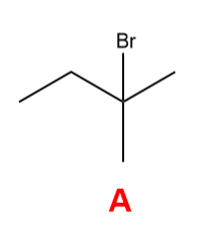
Markovnikov’s Rules
the H attaches to the C with fewer alkyl substituents and the X attaches to the C with more alkyl substituents in electrophilic addition of HX to an alkene
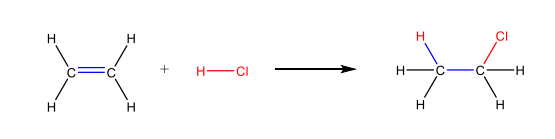
Regiospecific
reaction where only one structural isomer is produced
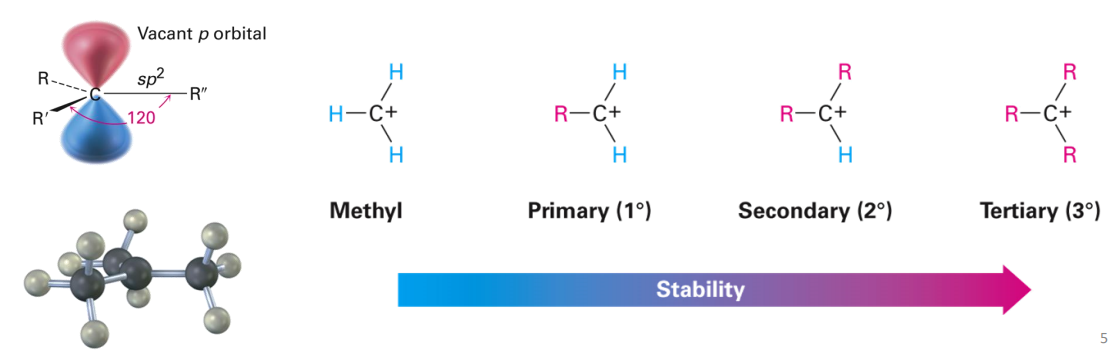
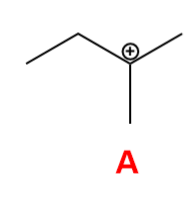
Carbocation Structure and Stability
Carbocations are trigonal planar, with three bonds and one empty p orbital
Carbocation stability increases with bonds to alkyl groups
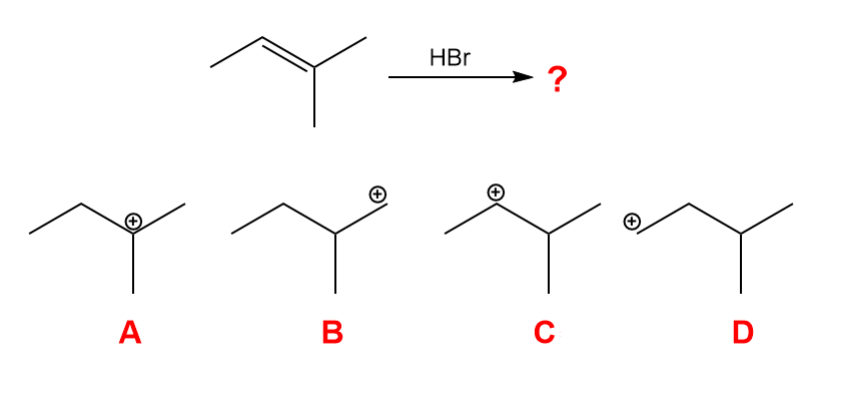
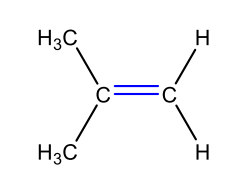
What is the carbocation intermediate that is formed in the following reaction?
A
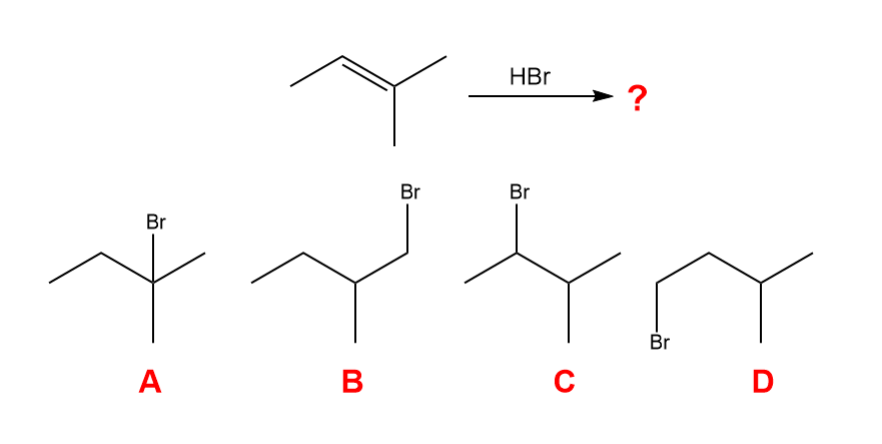
What is the major organic product that is formed in the following reaction?
A
Addition of Water to Alkenes
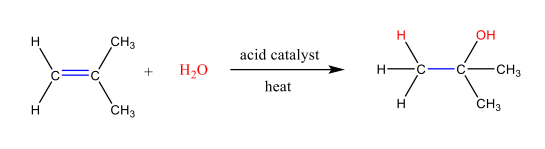
Addition of Water to Alkenes: Follows Markovnikov’s Rule
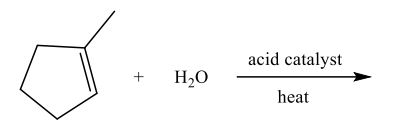
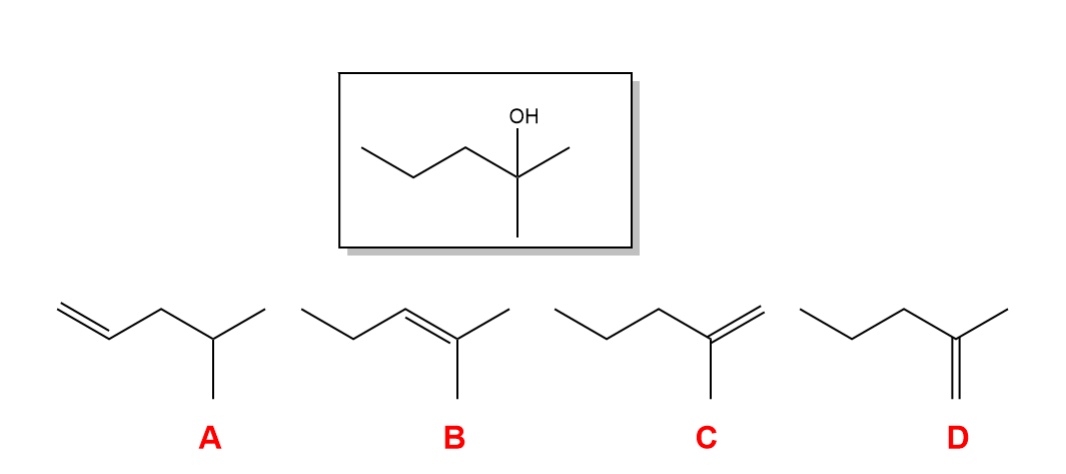
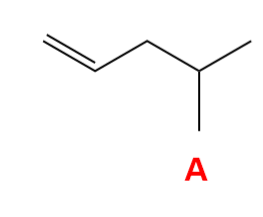
Select the alkene that will NOT produce the following product upon a hydration reaction.
A
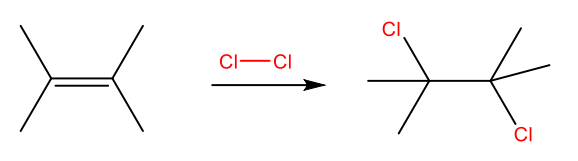
Halogenation: Addition of X2 to an Alkene
Bromine and chlorine add readily to yield 1,2-dihaloalkanes.
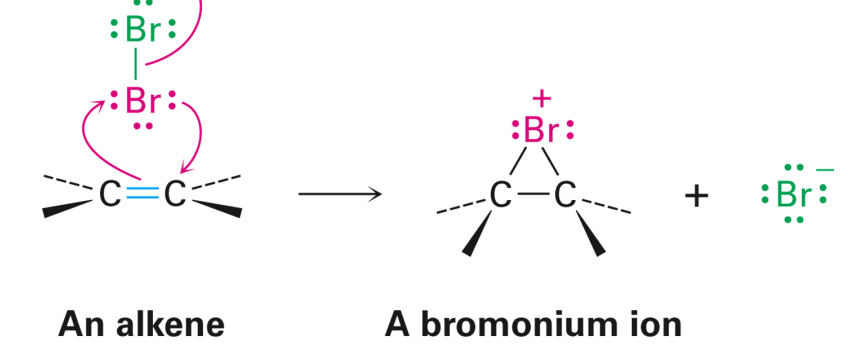
The Mechanism: Halogenation
Br+ adds to the alkene to produce a bromonium ion and bromide ion
The bromide then attacks from the opposite side of bromonium ion
Anti addition
Trans product is the major organic product
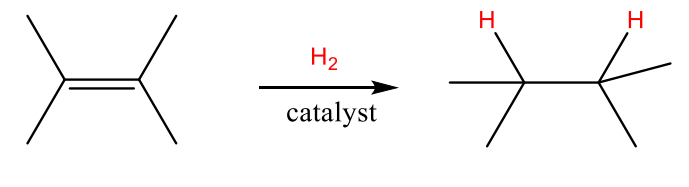
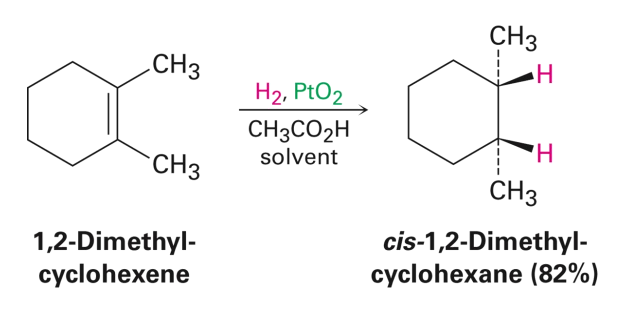
Hydrogenation to Akenes
• Hydrogen can be added catalytically over an alkene in the presence of a catalyst (typically Pd or Pt)
Produces only the cis product
Reduction reaction: Addition of hydrogen (or removal of oxygen) from a molecule
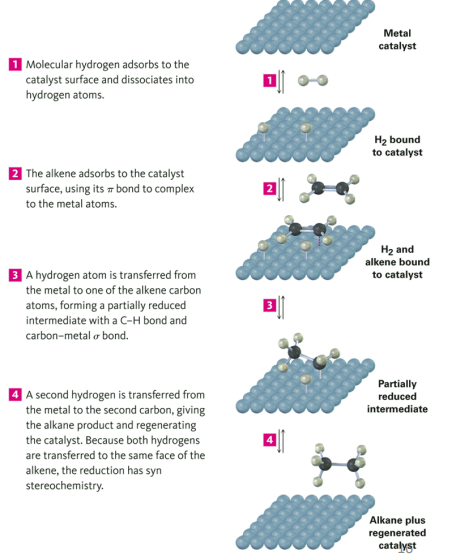
The Mechanism: Hydrogenation
Example of syn addition
Hydrogenation example
Oxidation of Alkenes: Epoxidation
Oxidation = addition of oxygen
Epoxide is a cyclic ether (oxirane), where there is one oxygen atom in a three-membered ring
Epoxide Applications
Cholesterol is a steroid that can be biosynthesized in living organism from a squalene intermediate
Squalene is a triterpene that is found in plants and animals
It was first discovered in shark liver oil
Squalene is also used in cosmetics, as it is a great lubricant
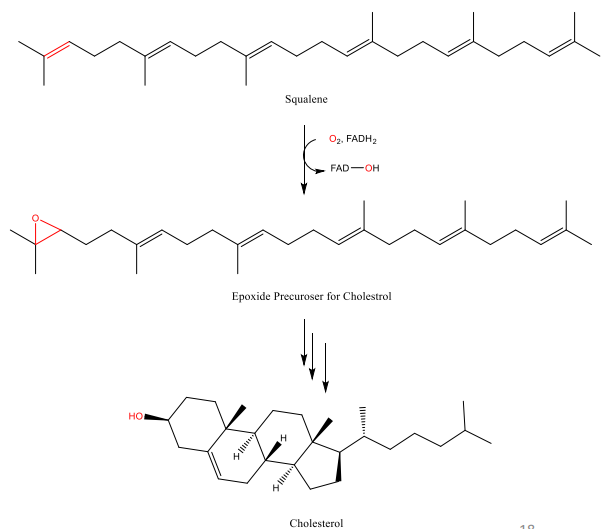
Epoxide Applications
Epoxy resins usually have starting monomers that contain an epoxy group.
The other monomer is an amine which acts as a hardener.
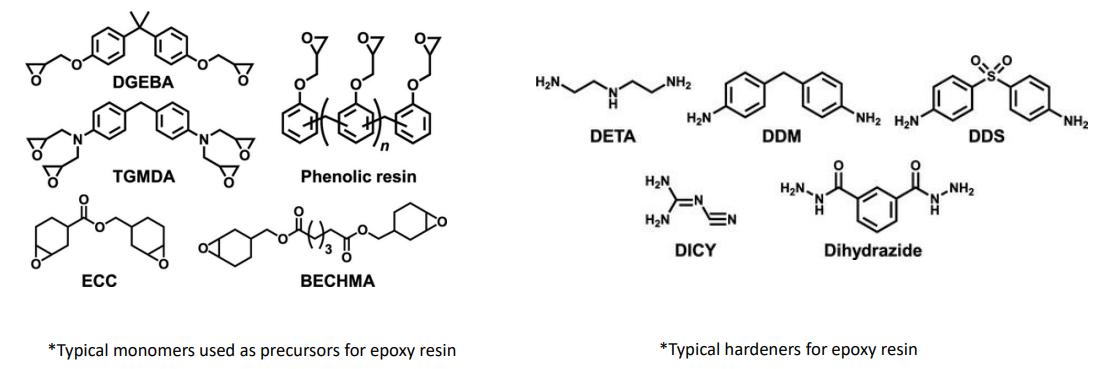
Epoxide Resins
Epoxy resins usually have starting monomers that contain an epoxy group.
Used in making jewelry, coasters, tables, fishing lures, and many other materials
Alkynes and their Reactions
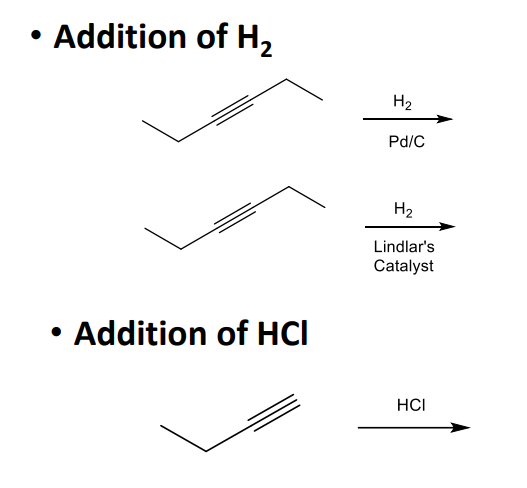
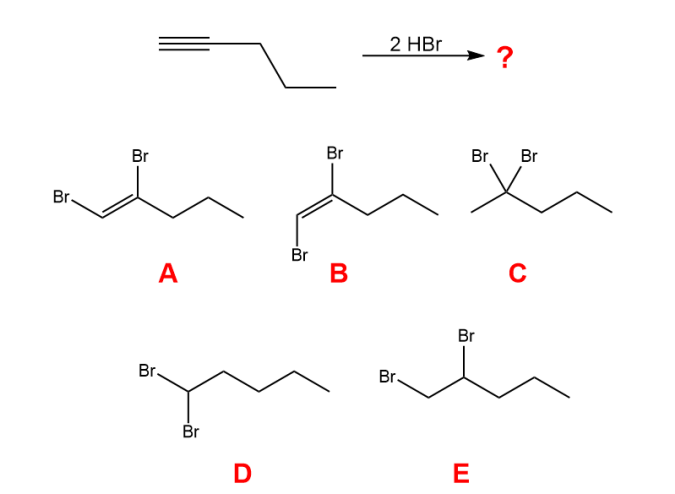
What is the major organic product of the following reaction?
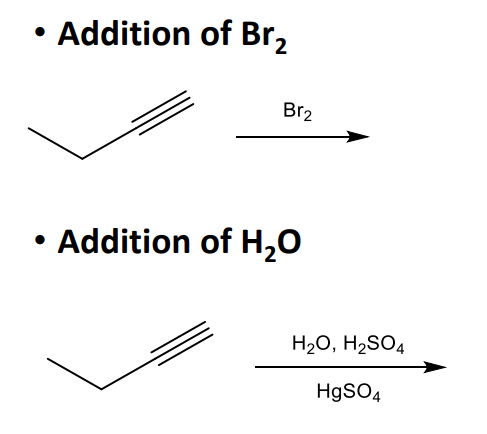
Alkynes and their Reactions cont.
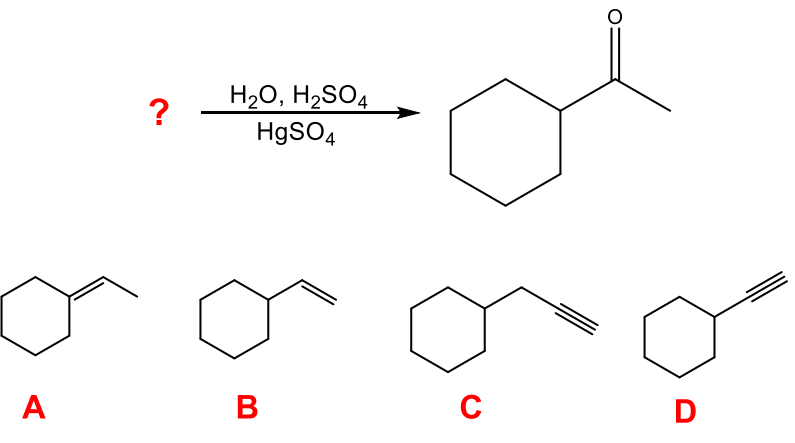
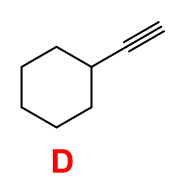
What starting material will produce the following product in the given set of conditions below?
Alkyne: Formation of Acetylide Anions
Acetylide anions can be formed from the reaction of a terminal alkyne with sodium amide.
Acetylide anions are good bases and good nucleophiles.