Chapter 21 Reactions
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
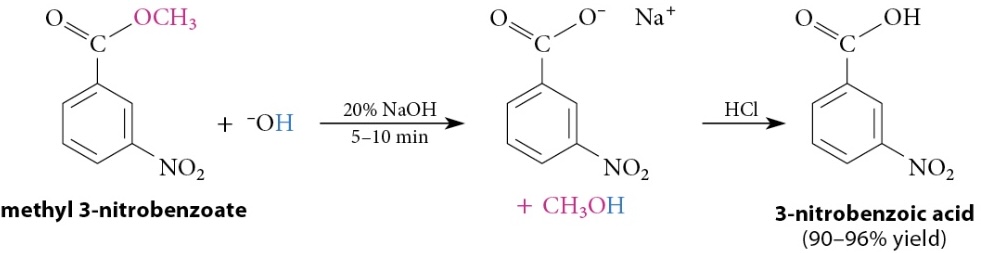
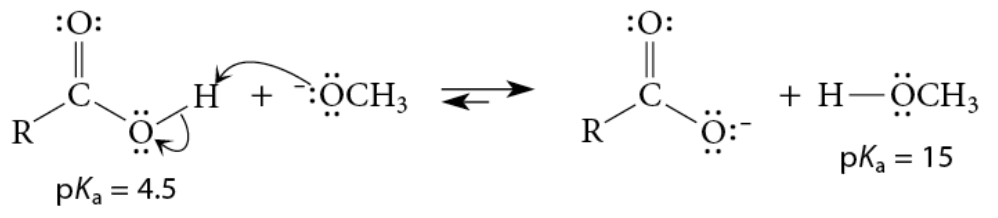
Base-Promoted Hydrolysis (Saponification) Esters (M)
Reaction
Cleavage reaction with hydroxide ion to yield a carboxylate salt and an alcohol
Strong acid required in second step
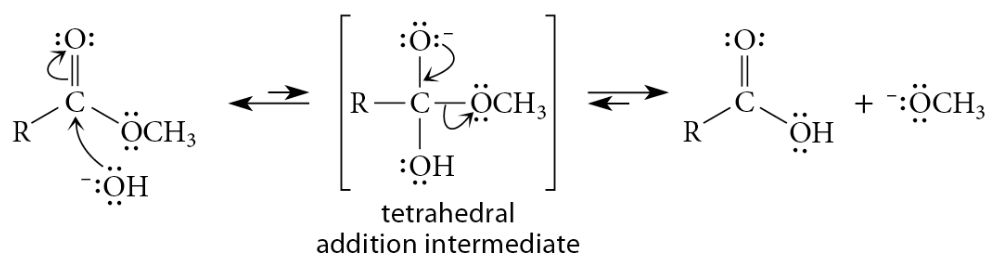
Mechanism
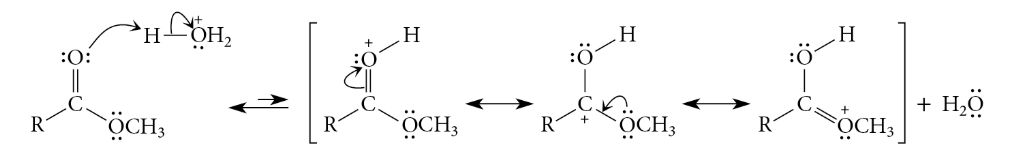
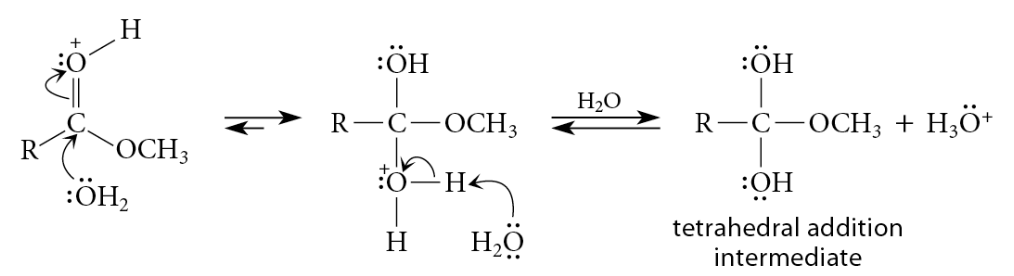
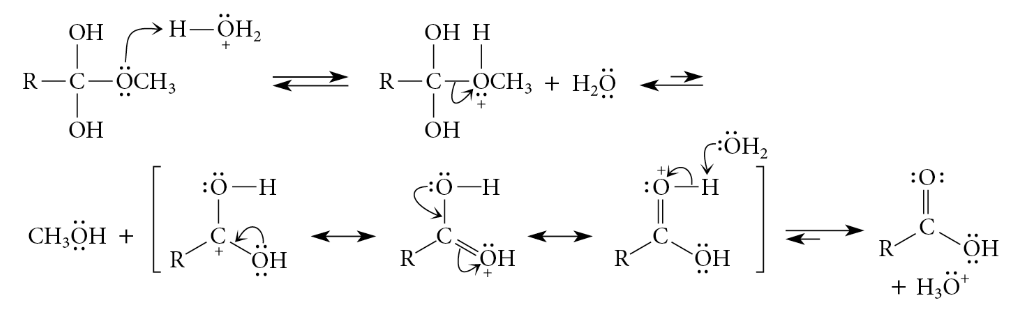
Acid-Catalyzed Esters Hydrolysis
Reaction
Esters can be hydrolyzed to carboxylic acids in aqueous solutions of strong acids
Reverse of the mechanism of acid-catalyzed esterification
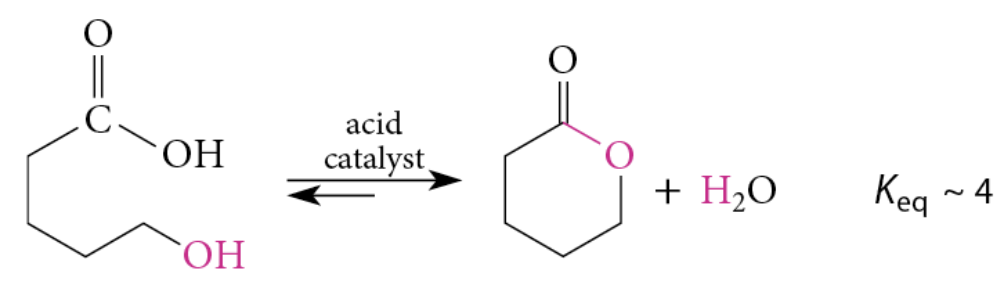
Hydrolysis and Formation of Lactones
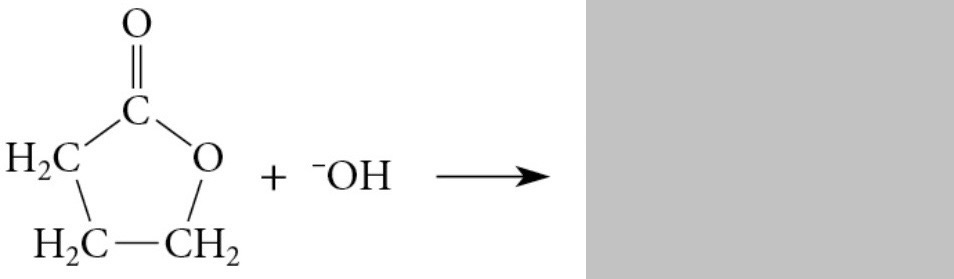
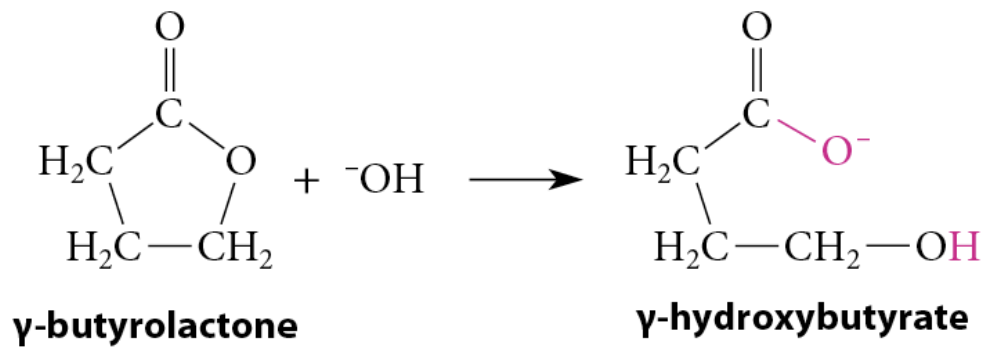
Reaction info
Saponification converts a lactone completely into a carboxylate salt of the corresponding hydroxy acid
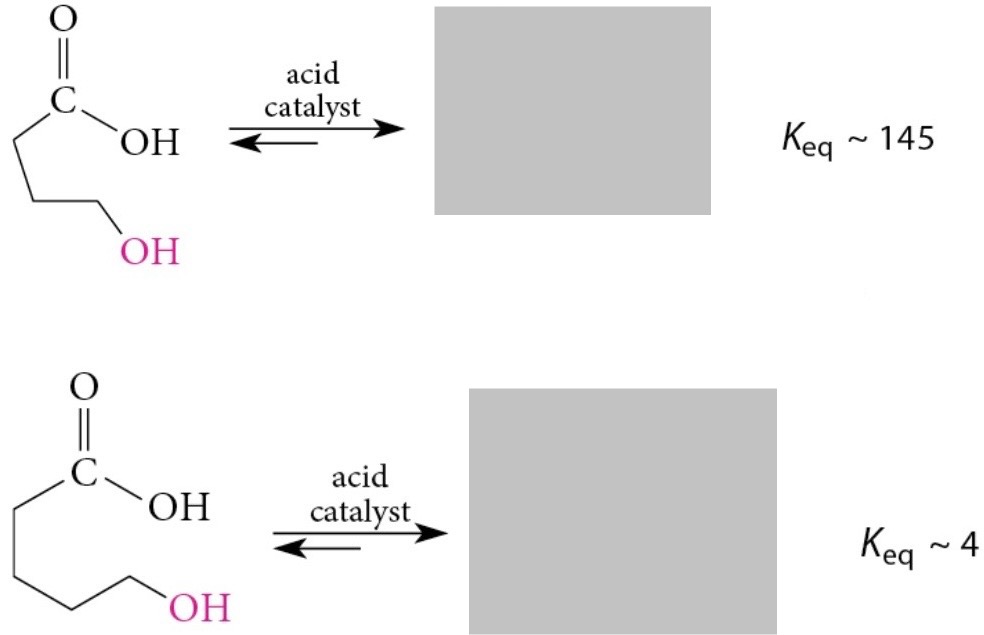
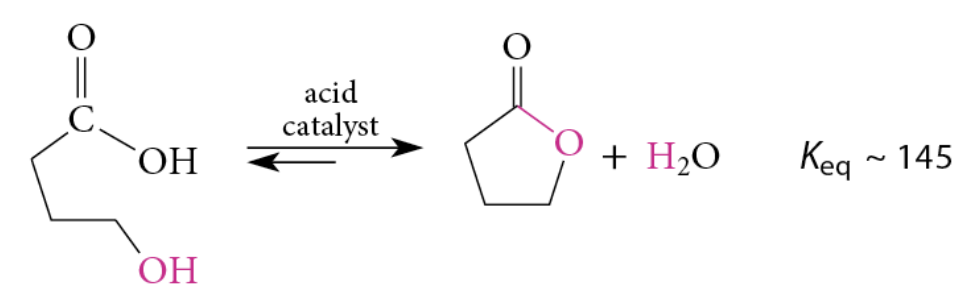
Acidification → hydroxy acid
Hydroxy acid + acidic solution → lactone
Lactones with 5 and 6 membered rings are favored
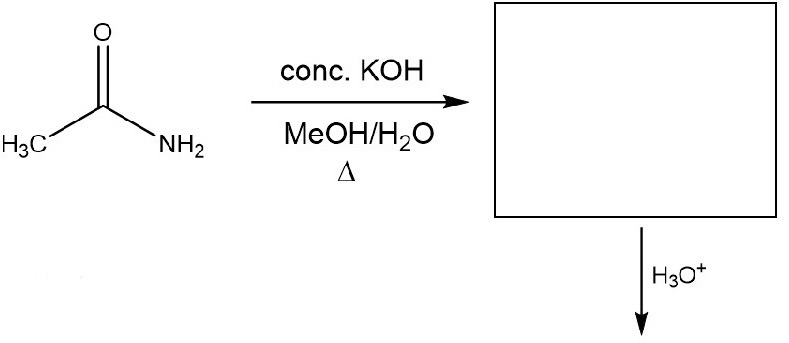
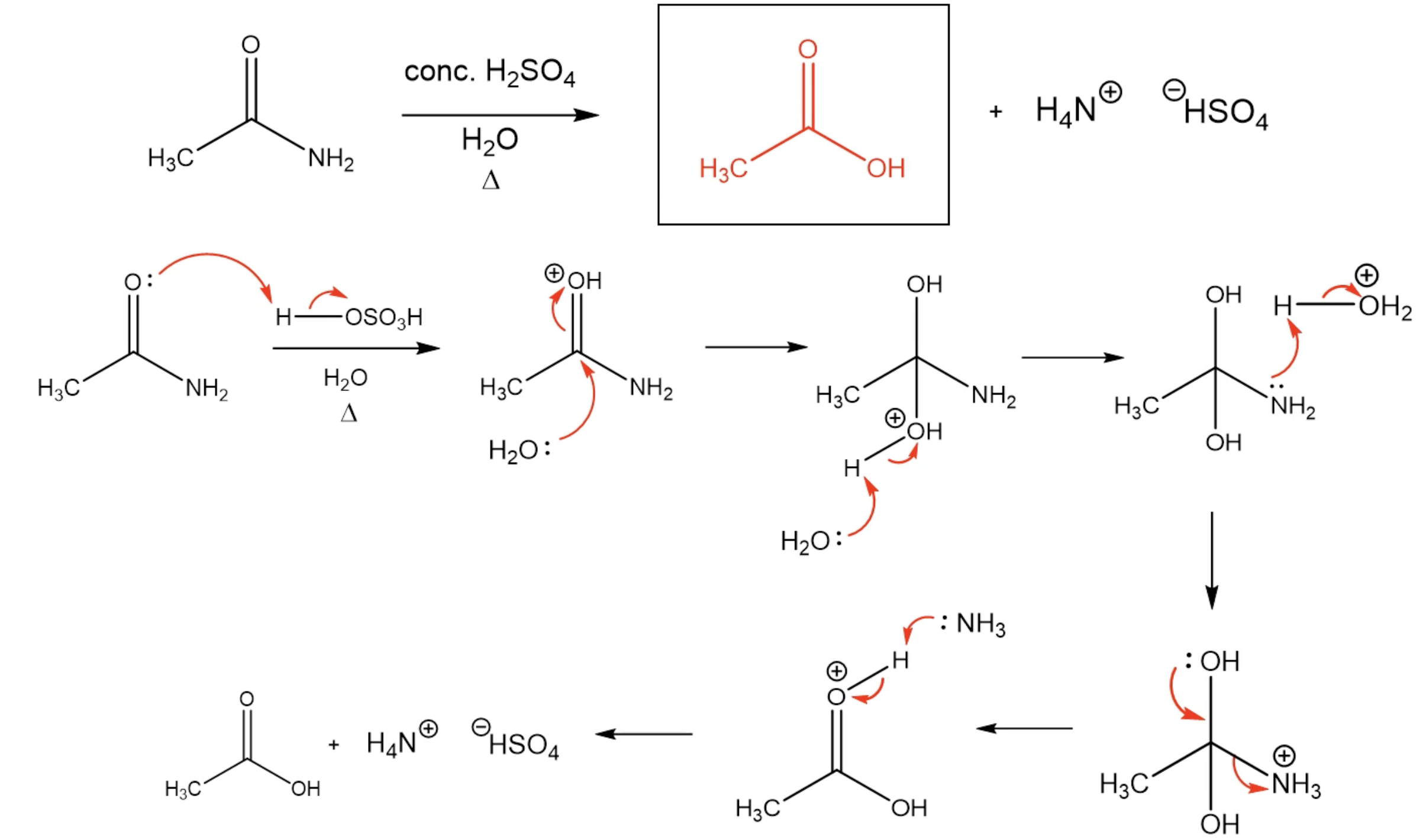
Hydrolysis of Amides (M)
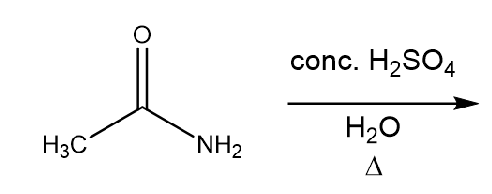
Reaction (With Acidic Solution)
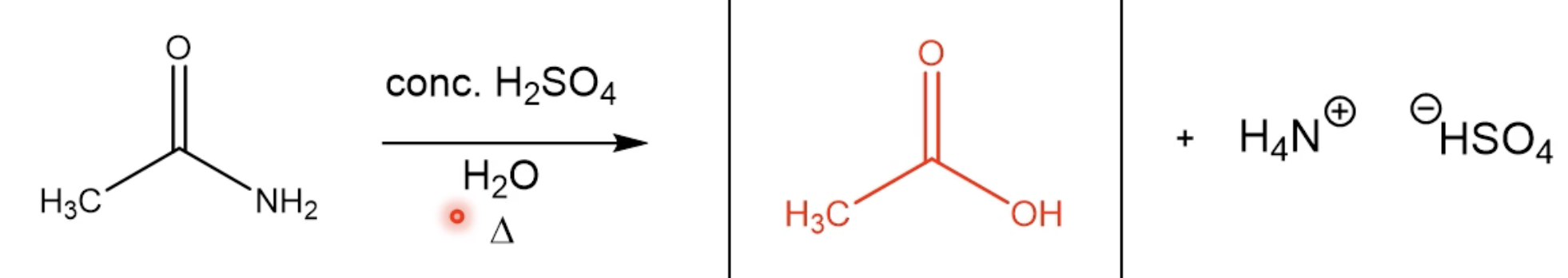
Mechanism
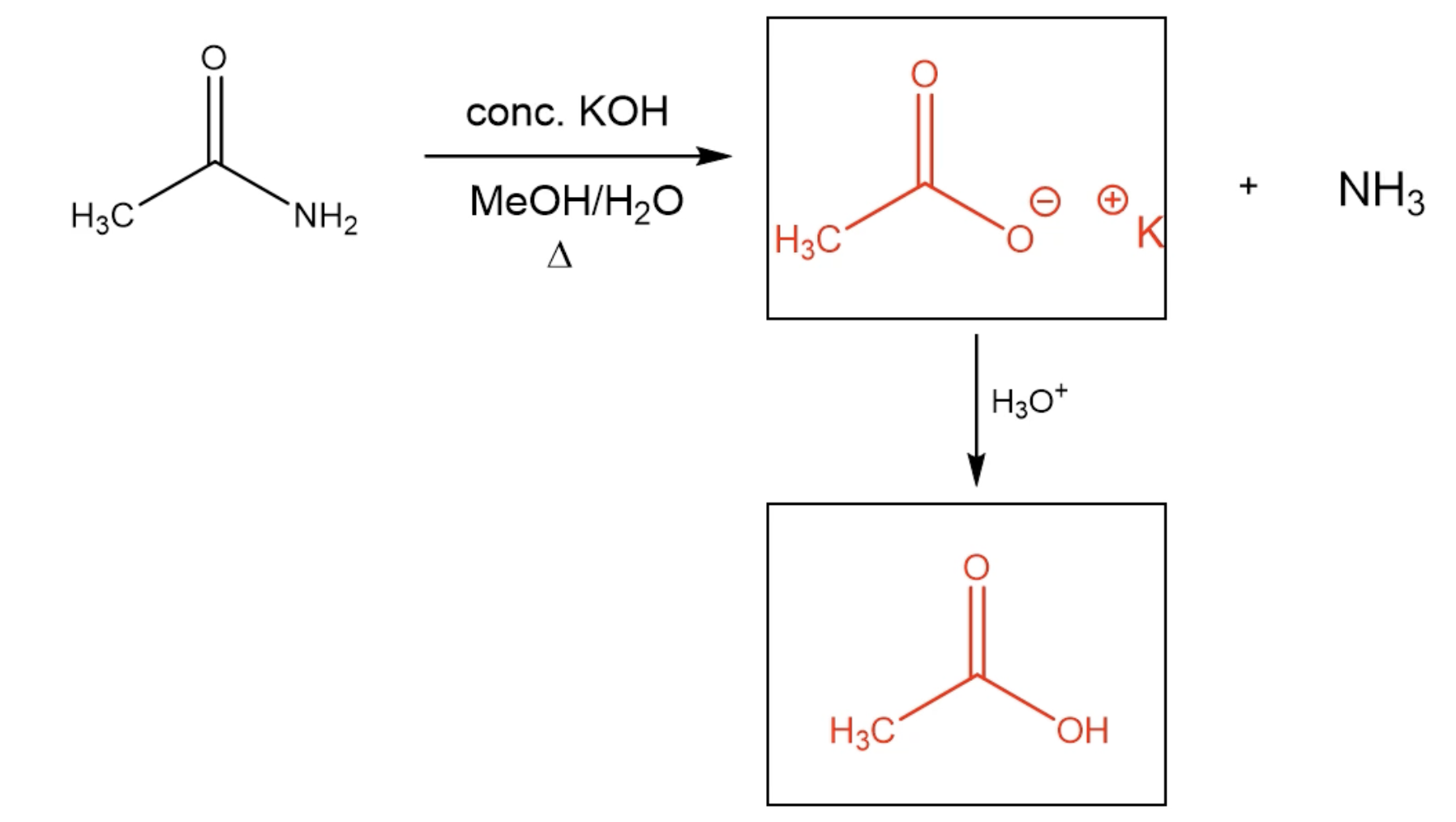
Reaction (With Basic Solution)
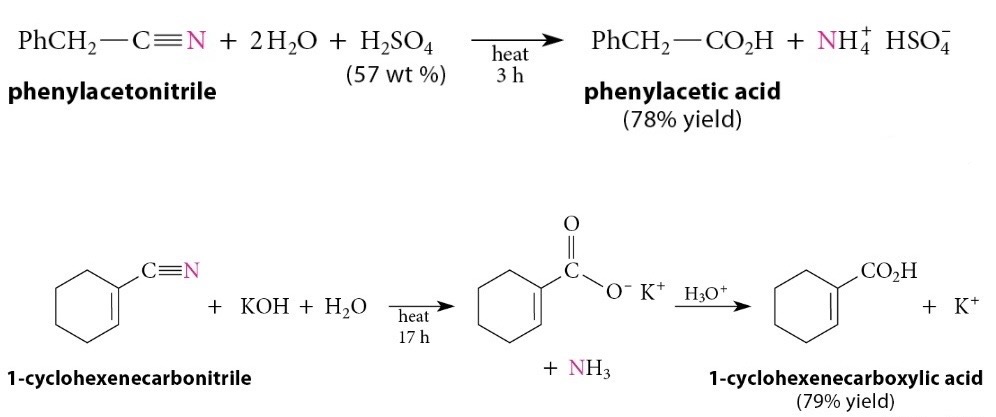
Hydrolysis of Nitriles
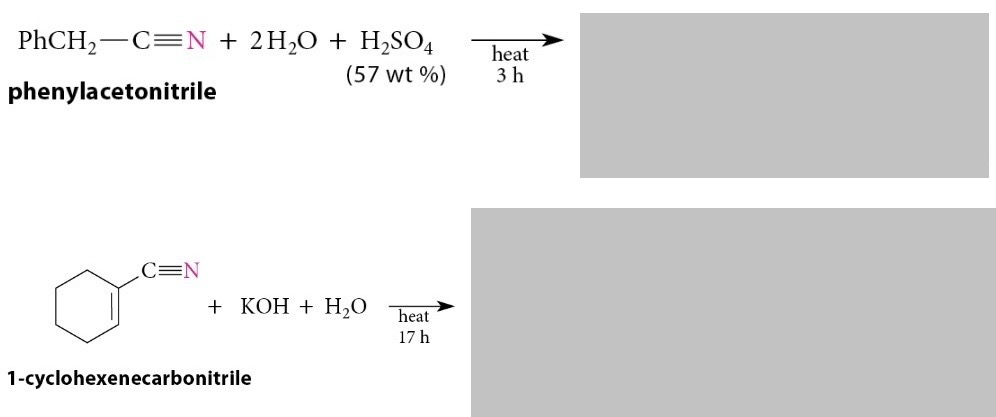
Reaction
Nitriles are hydrolyzed to carboxylic acids and ammonia by heating them in strongly acidic or strongly basic solution
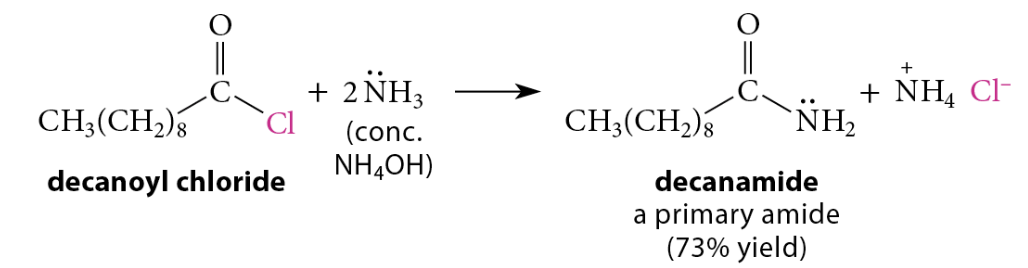
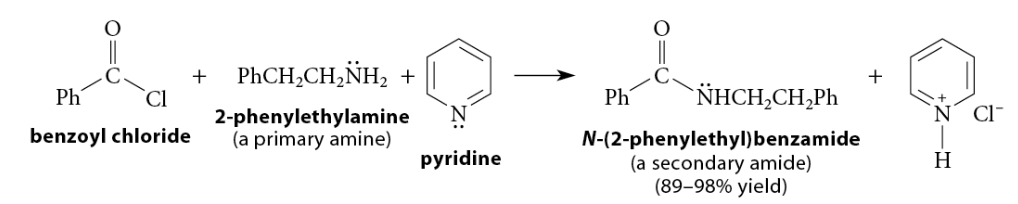
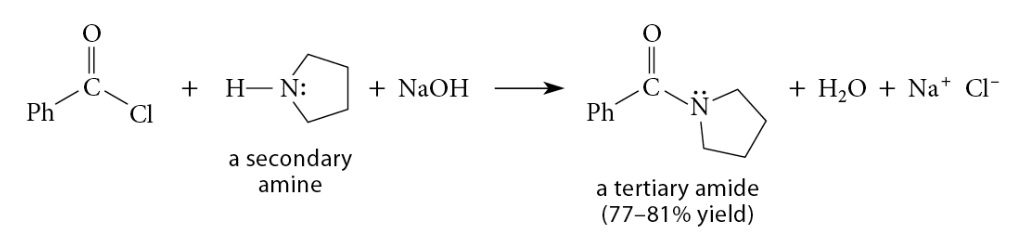
Reactions of Acid Chlorides with Ammonia and Amines
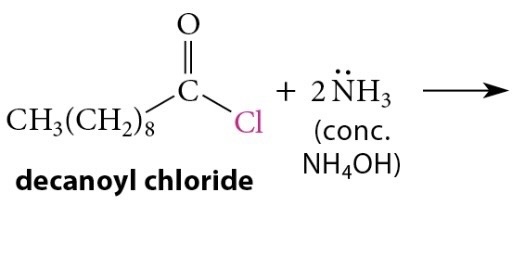

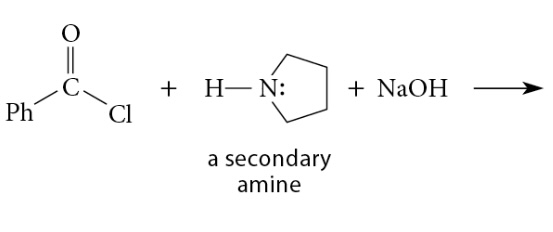
Reaction
Reaction of acid chloride with ammonia → primary amide
Reaction of acid chloride with primary amine → secondary amide
Reaction of acid chloride with secondary amine → tertiary amide
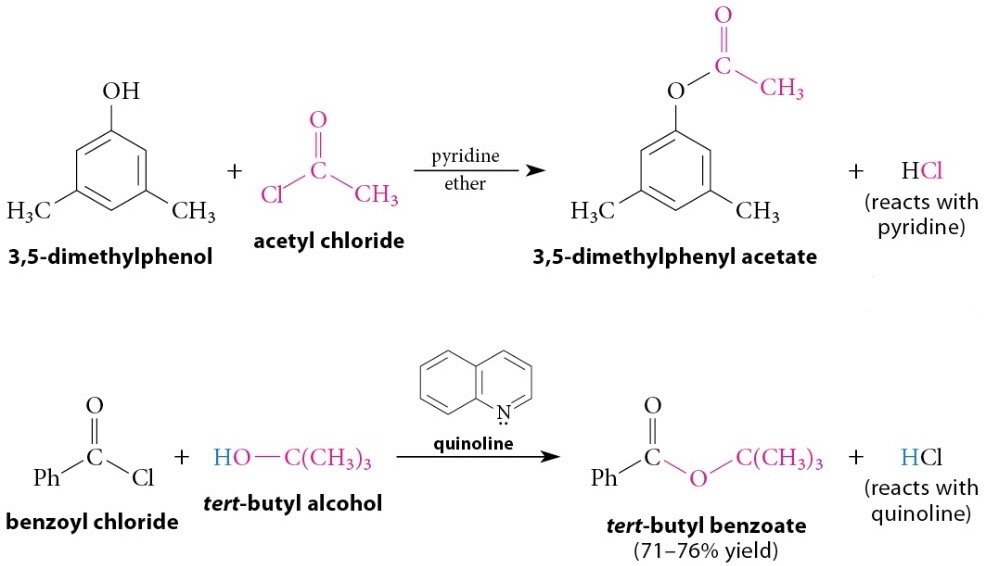
Reaction of Acid Chlorides With Alcohols and Phenols
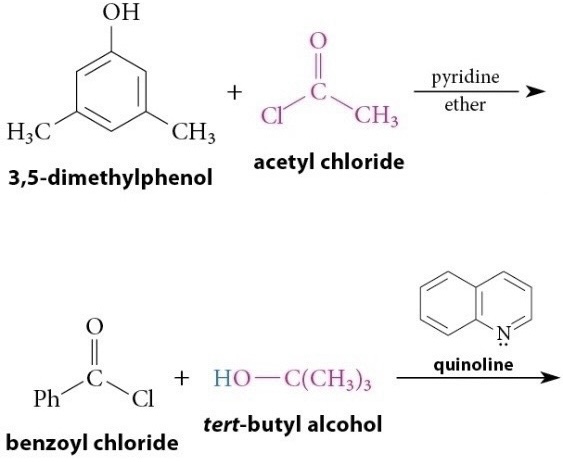
Reaction
Esters of tertiary alcohols and phenols, which cannot be prepared by acid-catalyzed esterification, can be prepared by this method
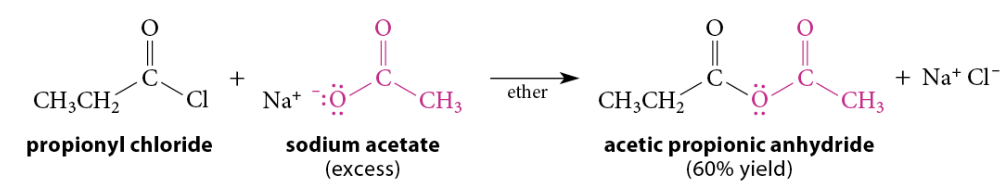
Reaction of Acid Chlorides With Carboxylate Salts
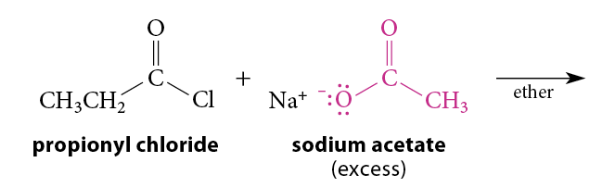
Reaction
Acid chlorides are reactive enough to react with carboxylate salts to give anhydrides
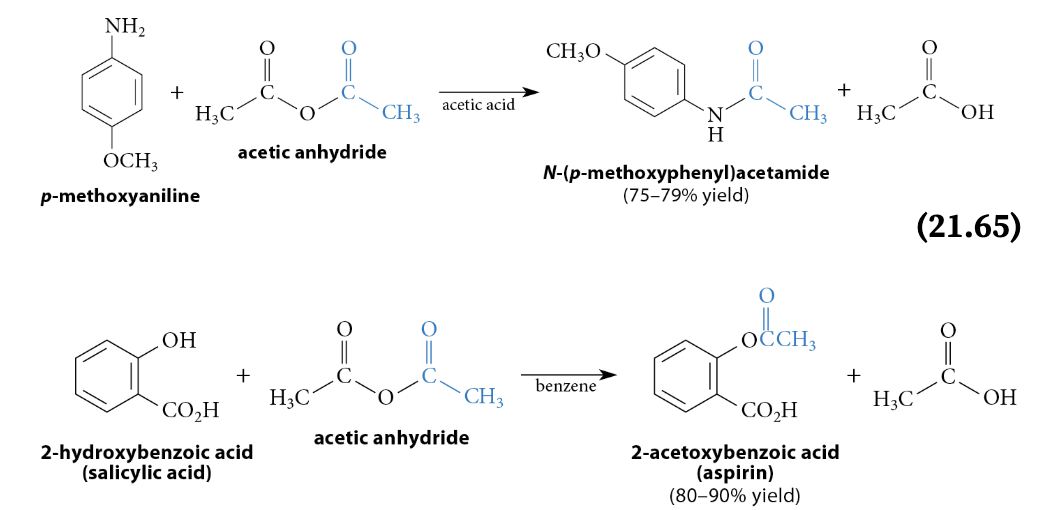
Reactions of Anhydrides With Nucleophiles
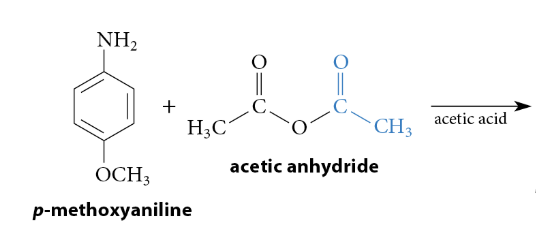
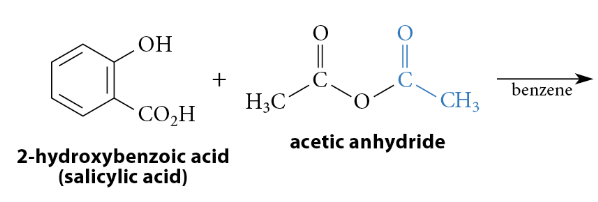
Reaction
The reaction with an amine yields an amide, the reaction with an alcohol yields an ester, etc
Reactions of Esters With Nucleophiles
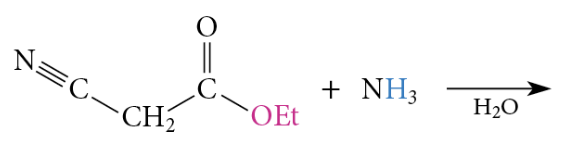
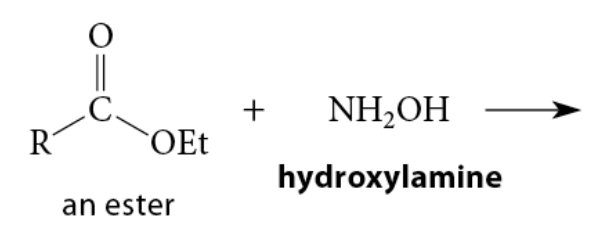
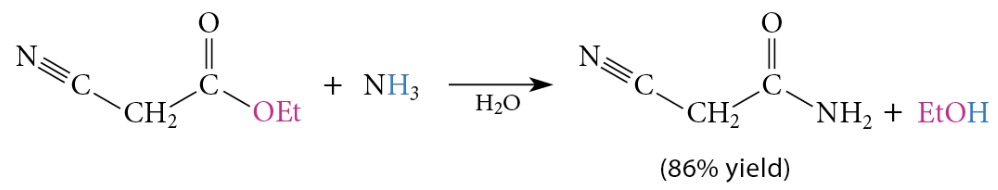
The reaction of esters with ammonia or amines yields amides
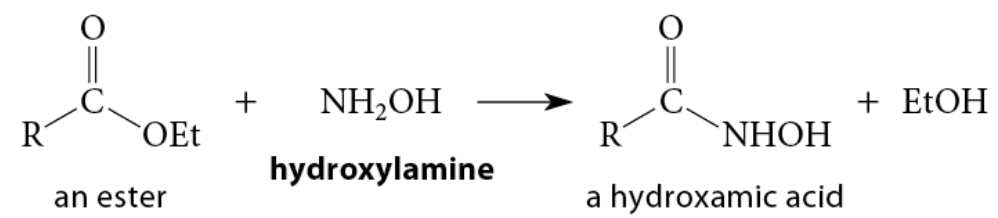
The reaction of esters with hydroxylamine gives N-hydroxyamides; these compounds are known as hydroxamic acids
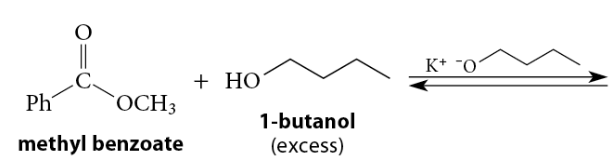
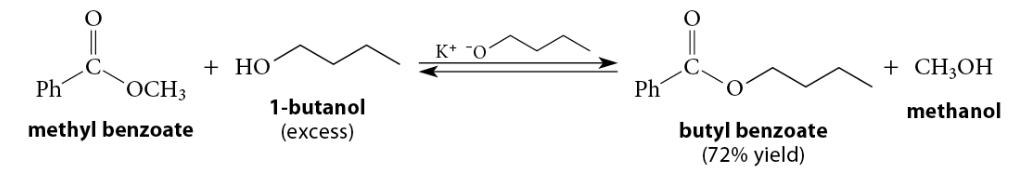
When an ester reacts with an alcohol under acidic conditions, or with an alkoxide under basic conditions, a new ester is formed
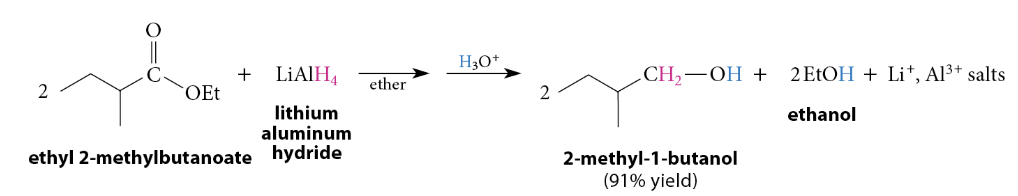
Reduction of Esters to Primary Alcohols
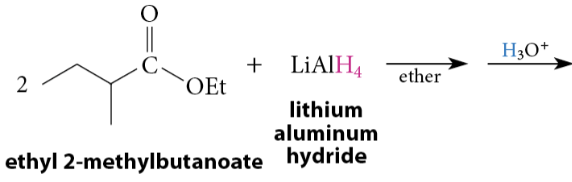
LiAlH4 reduces all carboxylic acid derivatives → primary alcohols
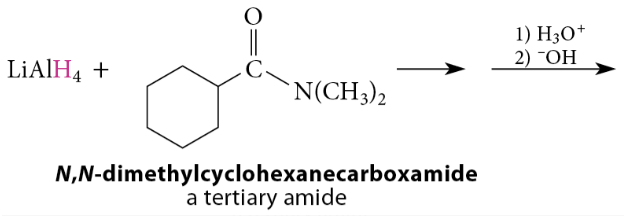
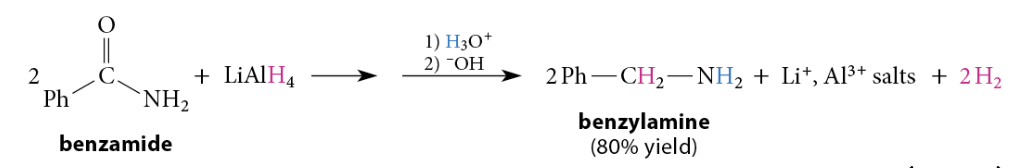
Reduction of Amides to Amines
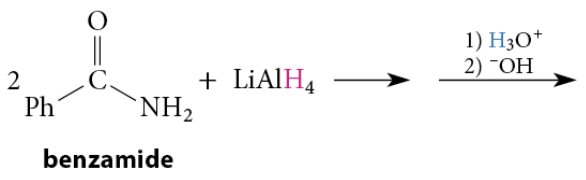
Amines are formed when amides are reduced with LiAlH4
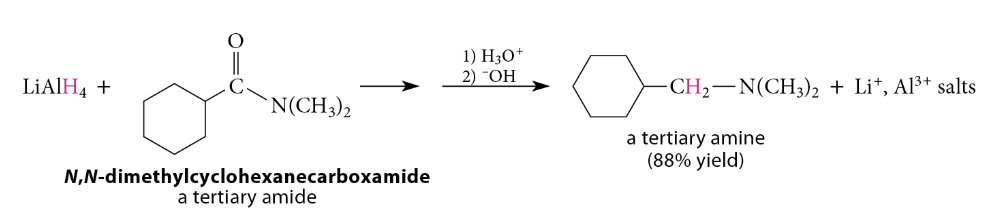
Amide reduction can be used not only to prepare primary amines from primary amides, but also to prepare secondary and tertiary amines from secondary and tertiary amides
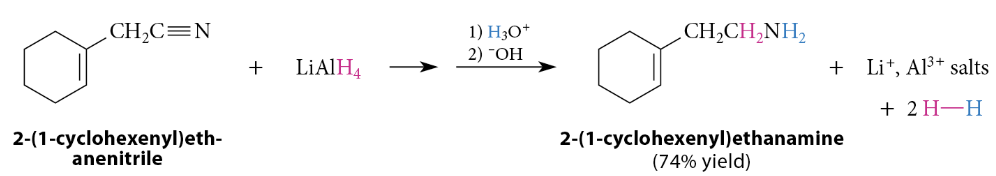
Reduction of Nitriles to Primary Amines
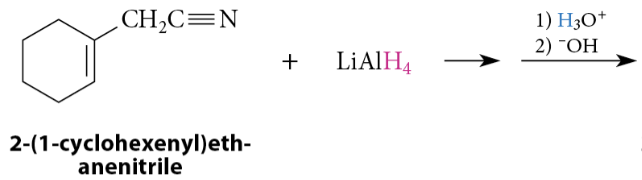
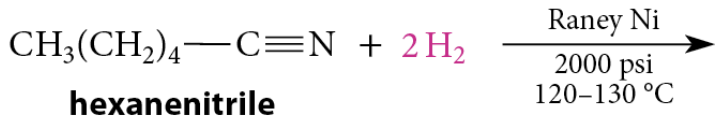
Nitriles are reduced to primary amines by reaction with LiAlH4
Nitriles are also reduced to primary amines by catalytic hydrogenation using Raney nickel
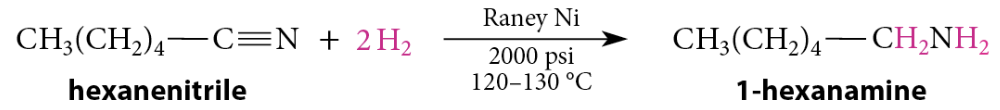
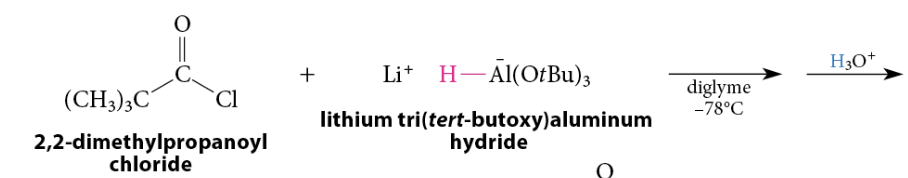
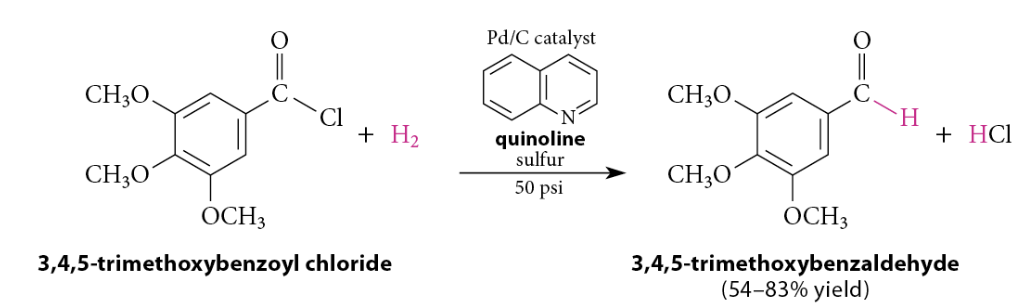
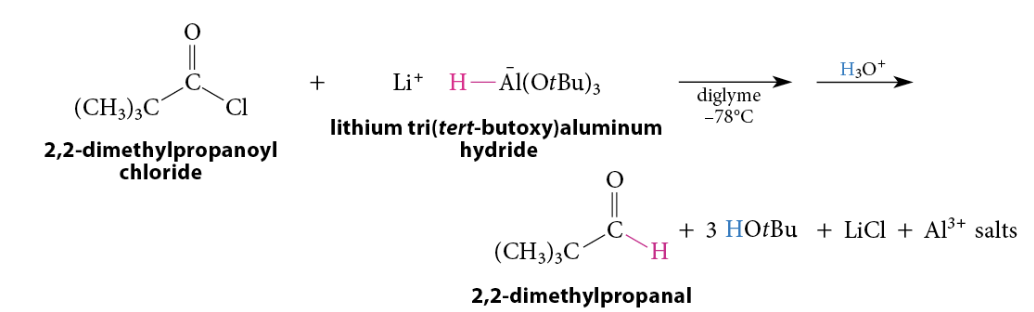
Reduction of Acid Chlorides To Aldehydes
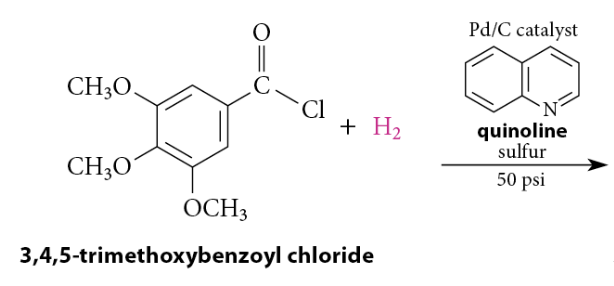
Rosenmund Reduction: the acid chloride is hydrogenated over a catalyst that has been deactivated, or poisoned, with an amine, such as quinoline, that has been heated with sulfur
The reaction of an acid chloride at low temperature with lithium tri(tert-butoxy)aluminum hydride
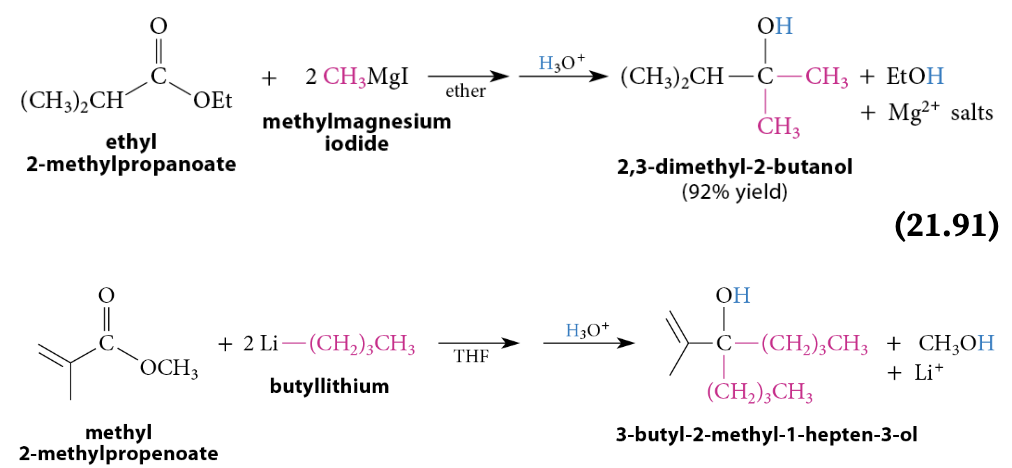
Reaction of Esters With Grignard and Organolithium Reagents
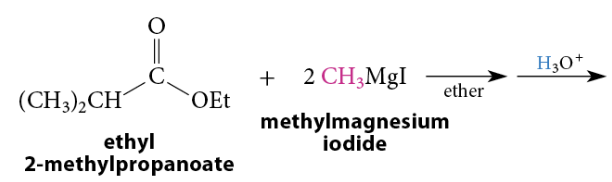
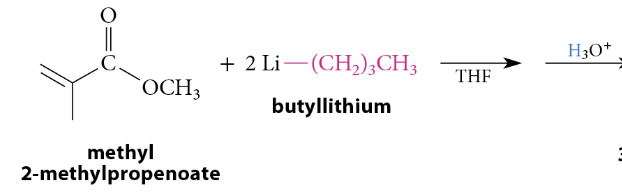
A tertiary alcohol is formed after protonolysis. A secondary alcohol is formed from esters of formic acid
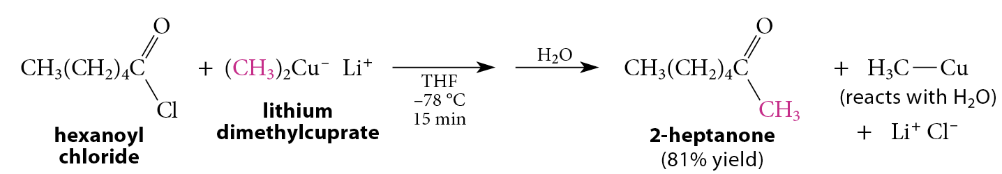
Reaction of Acid Chlorides with Lithium Dialkylcuprates
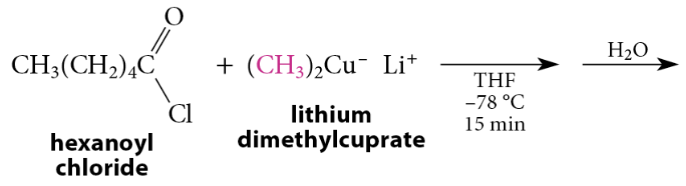
Lithium dialkylcuprates typically react readily with acid chlorides, aldehydes, and epoxides, very slowly with ketones, and not at all with esters. The reaction of lithium dialkylcuprates with acid chlorides gives ketones in excellent yield. Because ketones are much less reactive than acid chlorides toward lithium dialkylcuprates, they do not react further