U3(b): Synthesis of Organic Compounds
1/62
Earn XP
Description and Tags
U4: Organic Chemistry and Instrumental Analysis - (b) Synthesis of Org
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
Homolytic fission
results in the formation of two neutral radicals
occurs when each atom retains one electron from the σ covalent bond and the bond breaks evenly
normally occurs when non-polar covalent bonds are broken
Explain why heterolytic fission, rather than homolytic fission, is used for organic synthesis
Reactions involving homolytic fission tend to result in the formation of very complex mixtures of products, making them unsuitable for organic synthesis.
Reactions involving heterolytic fission tend to result in far fewer products than reactions involving homolytic fission, and so are better suited for organic synthesis.
Heterolytic fission
results in the formation of two oppositely charged ions
occurs when one atom retains both electrons from the σ covalent bond and the bond breaks unevenly
normally occurs when polar covalent bonds are broken
Heterolytic fission of CH3I
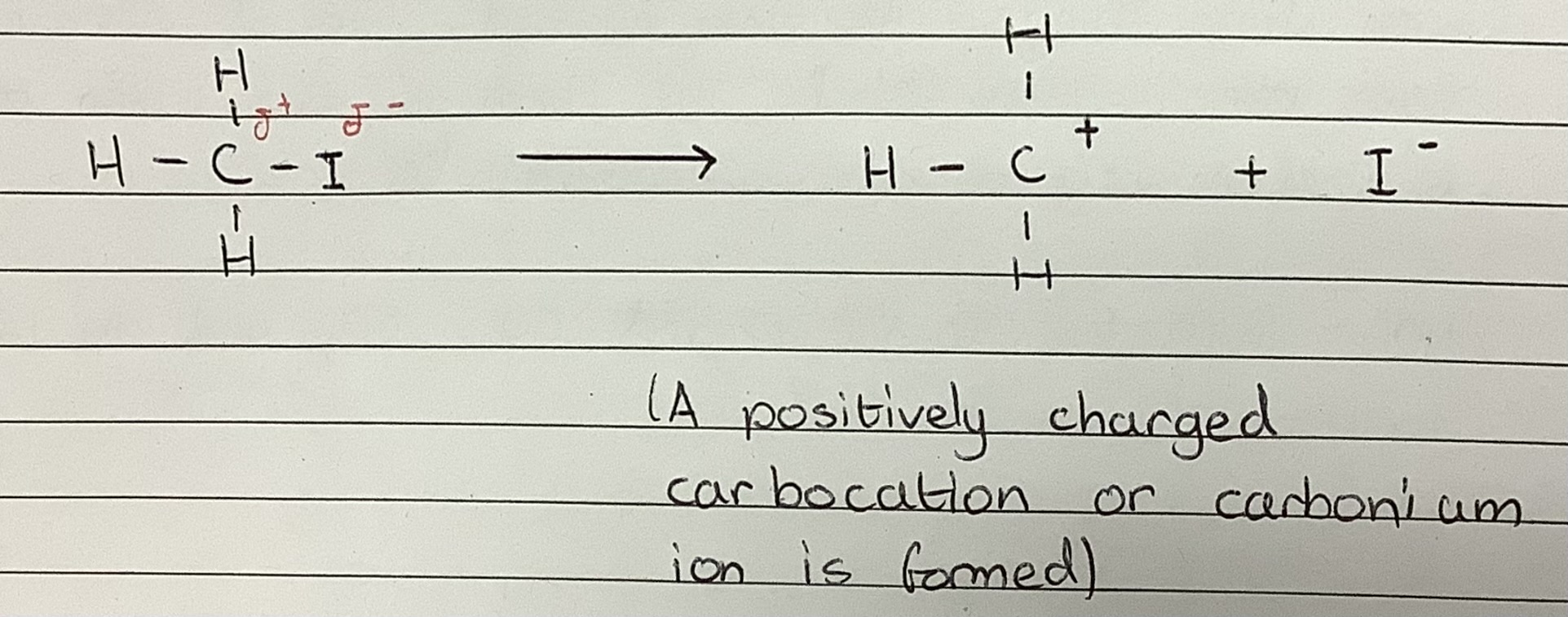
Carbocation
An ion with a positively charged carbon atom
Carbonion
An ion with a negatively charged carbon atom
Nucleophiles
negatively charged ions or neutral molecules that are electron rich
Examples of nucleophiles
Cl- , Br- , OH- , CN- , NH3 and H2O
Properties of Nucleophiles
attracted towards atoms bearing a partial (δ+) or full positive charge
capable of donating an electron pair to form a new covalent bond
Electrophiles
Positively charged ions or neutral molecules that are electron deficient
Examples of electrophiles
H+, NO2+, SO3
Properties of Electrophiles
attracted towards atoms bearing a partial (δ-) or full negative charge
capable of accepting an electron pair to form a new covalent bond
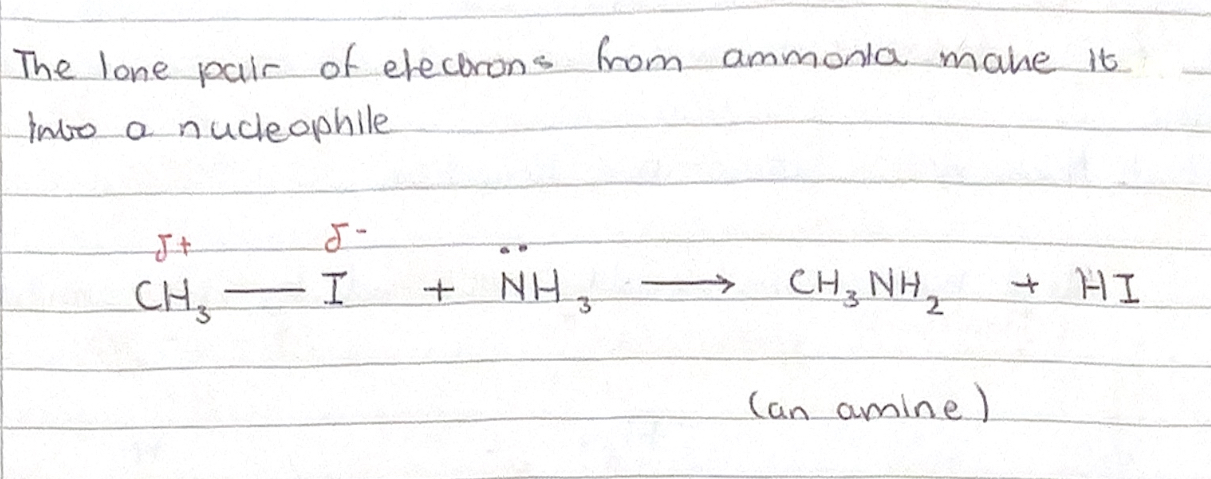
Explain why the ammonia molecule is a nucleophile
The presence of lone pair on the N atom
Haloalkanes (alkyl halides)
Substituted alkanes in which one or more of the hydrogen atoms is replaced with a halogen atom
Monohaloalkanes
Haloalkane containing only one halogen atom
Monohaloalkanes can be classified as:
primary, secondary or tertiary according to the number of alkyl
groups attached to the carbon atom containing the halogen atom
Monohaloalkane —?—> Alkene
ELIMINATION REACTION
using a strong base, such as potassium or sodium hydroxide in ethanol
Monohaloalkane + ? ——> Alcohol
NUCLEOPHILIC SUBSTITUTION REACTION
Monohaloalkane + aqueous alkali —> alcohol
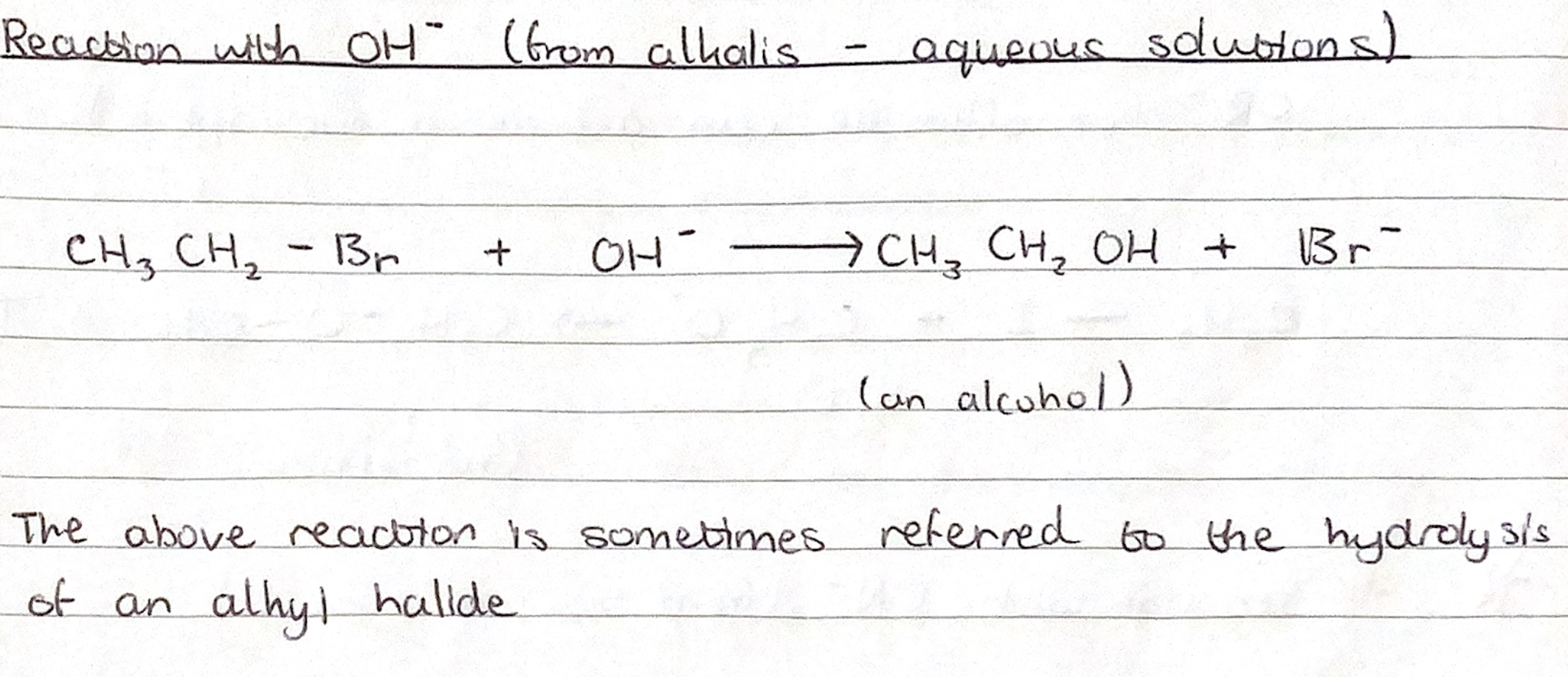
Monohaloalkane + ? ——> Ether
NUCLEOPHILIC SUBSTITUTION REACTION
Monohaloalkane + alcoholic alkoxide —> ether
Monohaloalkane + ? ——> nitrile
NUCLEOPHILIC SUBSTITUTION REACTION
Monohaloalkane + ethanolic cyanide —> nitriles
Chain length increased by one carbon atom
SN1 Reaction
Nucleophilic substitution reaction with one species in the rate determining step and occurs in a minimum of two steps via a trigonal planar carbocation intermediate
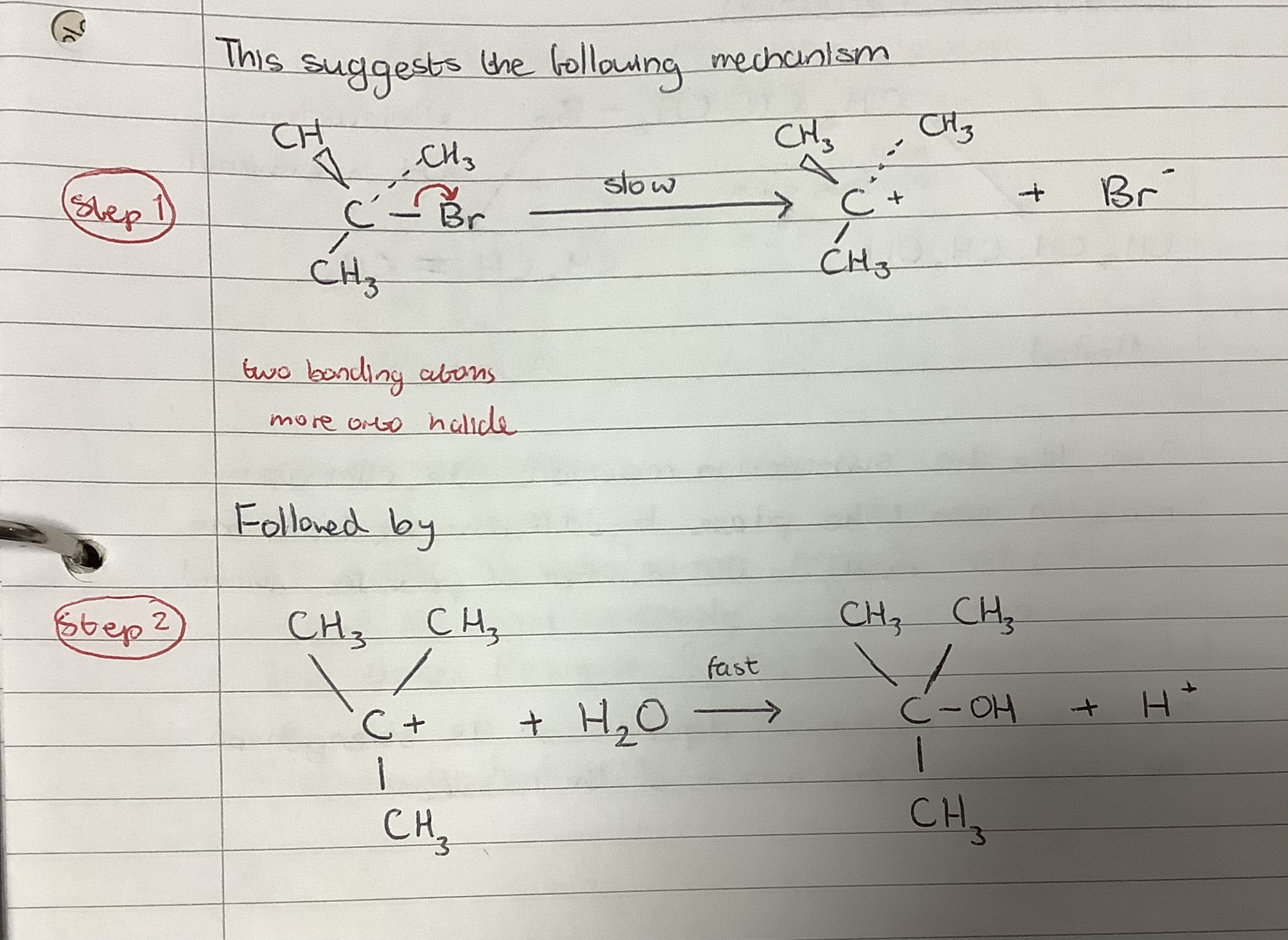
State the substitution reaction mechanisms for primary, secondary and tertiary haloalkanes and explain why they react by that reaction mechanism
Primary and secondary haloalkanes tend to react via SN2 mechanism
Tertiary haloalkanes tend to react via SN1 reactions due to steric hindrance of the side groups in the tertiary haloalkane blocking the attack of the nucleophile for the δ+on the carbon atom in the carbon to halogen bond. Alkyl groups off this carbon provide inductive stabilisation of the carbocation intermediate due to the repelling ability of the alkyl groups.
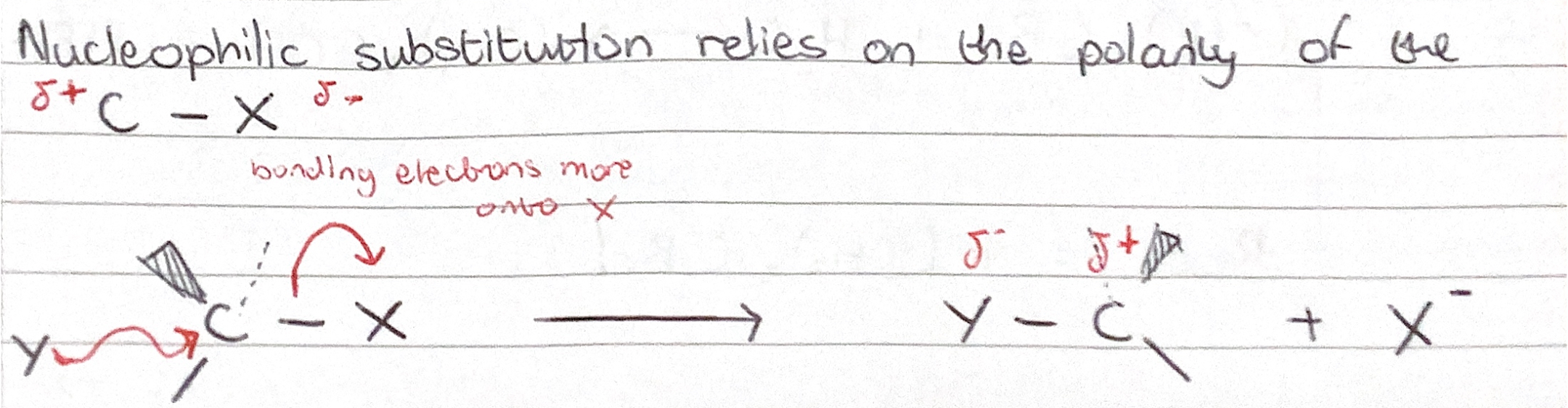
SN2 Reaction Mechanism
nucleophilic substitution reaction with two species in the rate determining step and occurs in a single step via a single five-centred, trigonal bipyramidal transition state
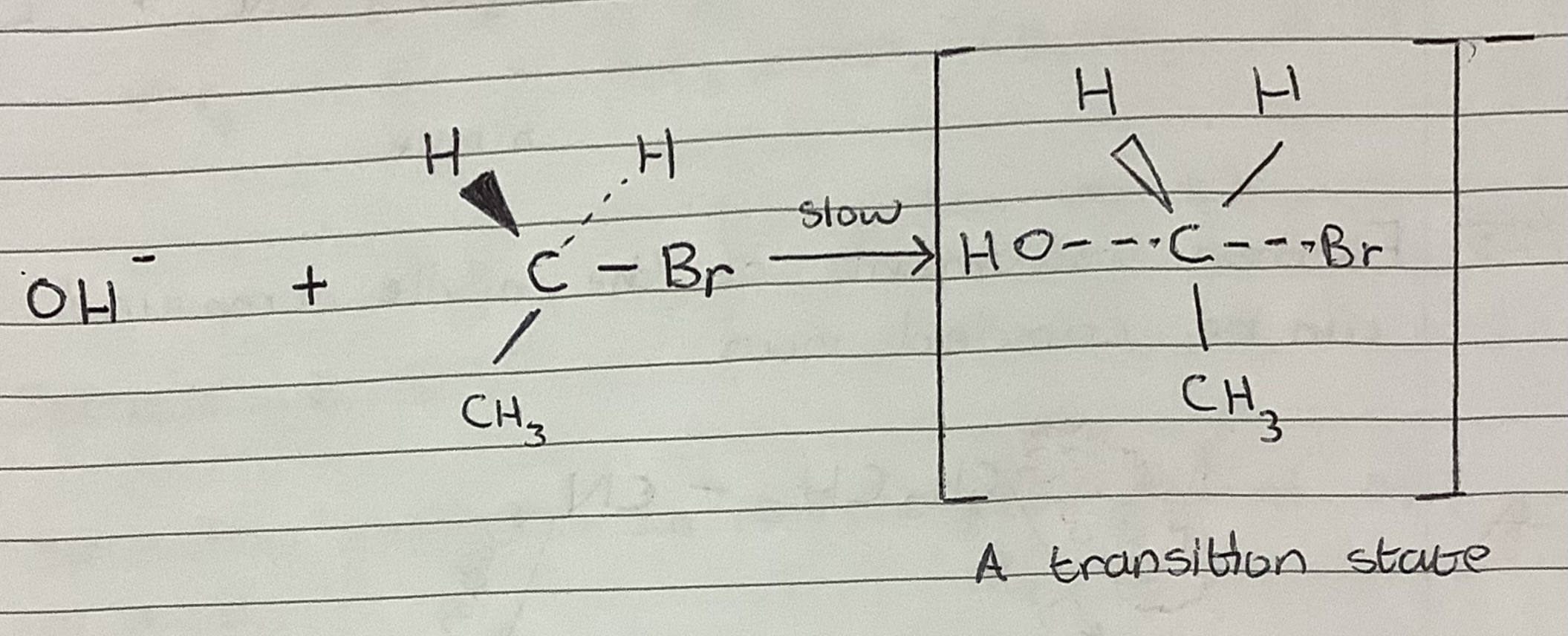
Nucleophilic substitution mechanism
Alcohols
substituted alkanes in which one or more of the hydrogen atoms is replaced with a hydroxyl functional group, –OH group
Alcohols can be prepared from:
haloalkanes by nucleophilic substitution with aqueous alkali
alkenes by acid-catalysed hydration (addition)
aldehydes and ketones by reduction using a reducing agent such as lithium aluminium hydride
Alcohol —?—> alkene
DEHYDRATION
Uses aluminium oxide, concentrated sulfuric acid or
concentrated phosphoric acid
Oxidation of primary alcohols
Primary alcohols can be oxidised to form aldehydes and then carboxylic acids
Oxidation of secondary alcohols
Secondary alcohols can be oxidised to form ketones. Ketones cannot be oxidised
Oxidising agents used to oxidise alcohols
Acidified dichromate
Acidified permanganate
Hot copper (II) oxide (used in synthesis reactions)
Formation of alcoholic alkoxides
Alcohols can react with some reactive metals such as potassium or sodium to form alcoholic alkoxides, which can then be reacted with monohaloalkanes to form ethers
Formation of esters
Esters can be formed by the reaction of alcohols with carboxylic acids using concentrated sulfuric acids or concentrated phosphoric acid as a catalyst
Esters can also be formed by reaction of alcohols with acid chlorides. Carboxylic acids can be converted to acid chlorides by reaction with SOCl3, PCl3 or PCl5. Although this is a two-step preparation, this gives a faster reaction than carboxylic acids and no catalyst is needed.
Hydroxyl groups in alcohols
Hydroxyl groups make alcohols polar, which gives rise to hydrogen bonding
Hydrogen bonding can be used to explain the properties of alcohols including boiling points, melting points, viscosity and solubility or miscibility in water
Ethers
Ethers can be regarded as substituted alkanes in which a hydrogen atom is replaced with an alkoxy functional group, -OR
They have the general general structure R' – O – R'', where R' and R'' are alkyl groups (oxygen bridging two alkyl groups together)
Naming ethers
Ethers are named as substituted alkanes. The alkoxy group is named by adding the ending ‘oxy’ to the alkyl substituent, and this prefixes the name of the longest carbon chain.
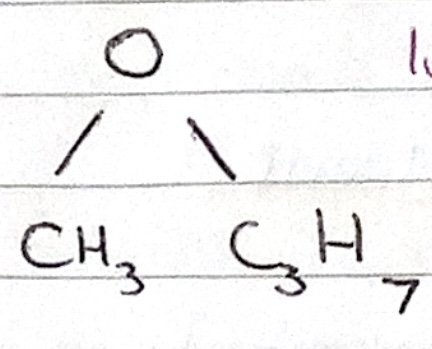
Name the ether
methoxypropane
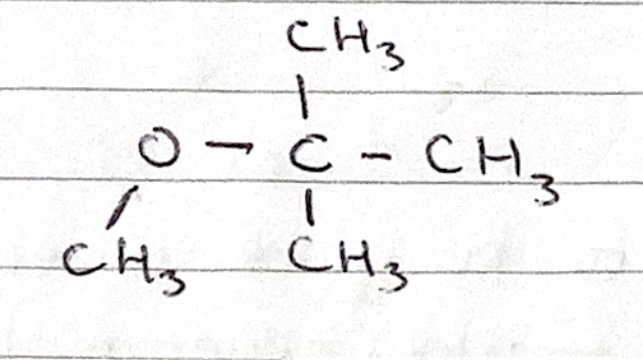
Name the ether
2-methoxy-2-methylpropane
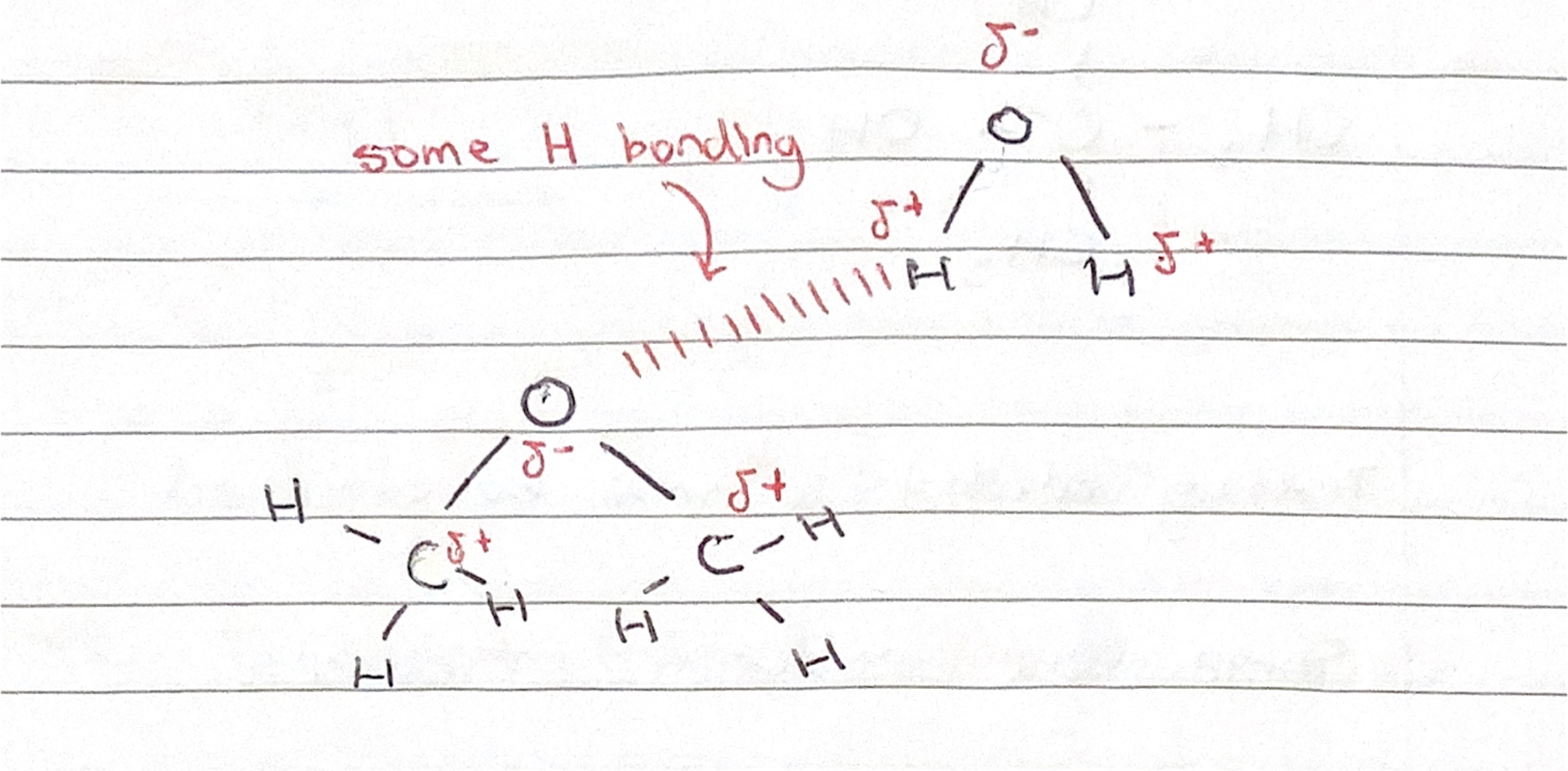
Hydrogen bonding in ethers
The lack of H bonding between ether molecules explains the relatively lower boiling points of ethers as compared with alcohols.
However, when mixed with water, the polarity of ethers gives rise to some H bonding.
Ethers with short alkyl groups do show some water solubility, however those with longer alkyl groups do not due to the hydrophobic nature of the longer non-polar hydrocarbon chain. ie. methoxymethane and methoxyethane are soluble in water; larger ethers are insoluble in water due to their increased molecular size.
Uses of ethers as solvents
Ethers are commonly used as solvents since they are relatively inert chemically and will dissolve many organic compounds, however, great care must be exercised in their use due to their:
Extreme volatility
Flammability
Potential for exposure to sunlight in resulting in their oxidation to peroxides, R-O-O-R, which are highly explosive. This is despite the tendency of ethers to be quite unreactive.
Halogenation of alkanes
Is a slow reaction
Requires the presence of UV light, usually from sunlight
Can be described as a substitution reaction
Is a chain reaction involving the formation of highly reactive atoms and free radicals, and consists of the steps initiation, propagation and termination (see reaction mechanism)
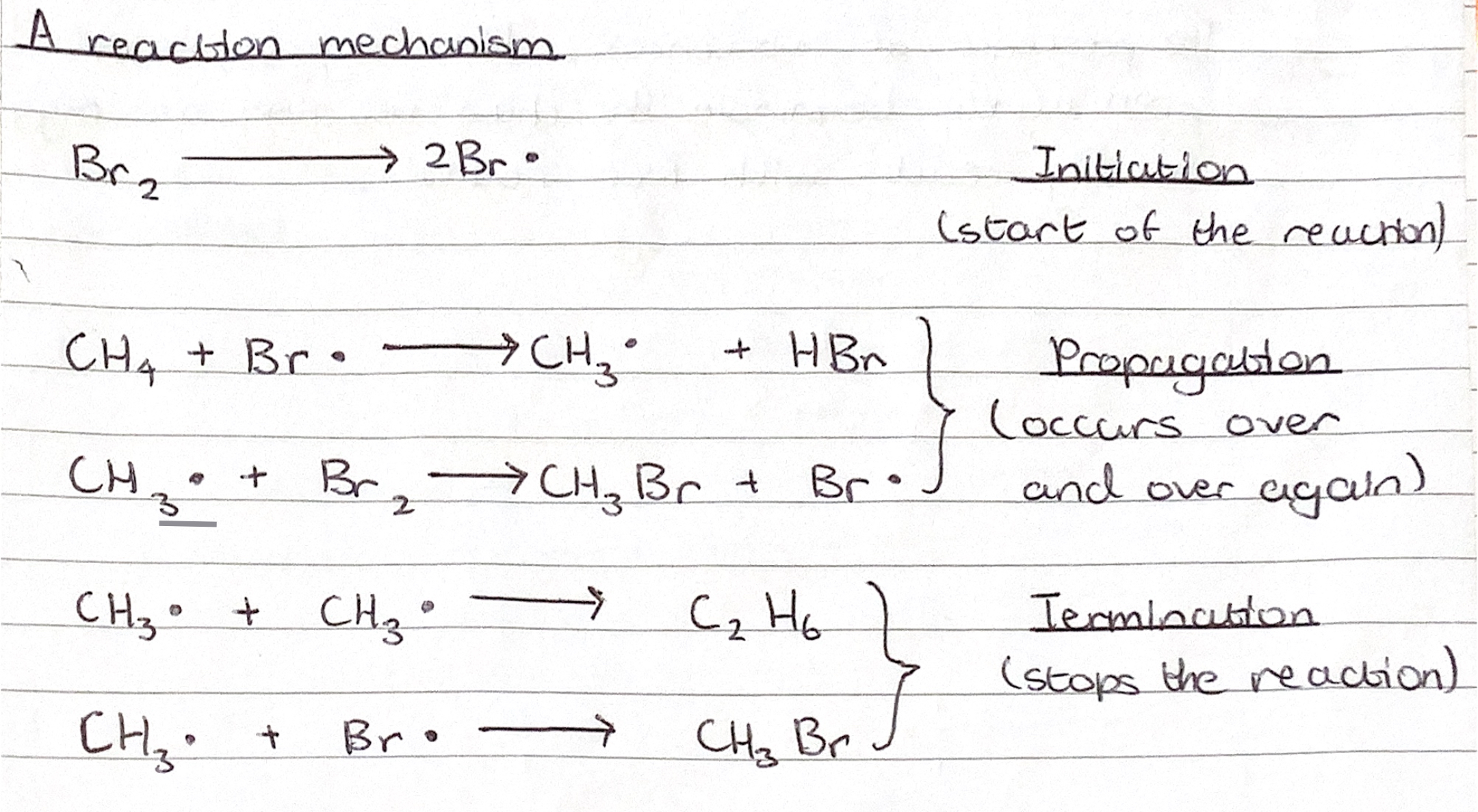
Explain how the type of halogen in a haloalkanes affects the reactivity of a haloalkane
The type of halogen bonded to the carbon affected the reactivity as for instance R-F bonds are very strong so very unreactive, whereas going down the halogen group, the R-X bond becomes weaker and so the alkyl halide becomes more reactive.
Alkyl halides are {nucleophiles/electrophiles}
Electrophiles
Monohaloalkane + ? ——> amine
NUCLEOPHILIC SUBSTITUTION
Monohaloalkane can react with ammonia to form an amine
Alkenes can be prepared by:
dehydration of alcohols using aluminium oxide, concentrated sulfuric acid (H2SO4) or concentrated phosphoric acid (H3PO4) (ELIMINATION)
base-induced elimination of hydrogen halides from monohaloalkanes
Alkenes take part in electrophilic addition reactions with:
hydrogen to form alkanes in the presence of a catalyst (hydrogenation)
halogens to form dihaloalkanes (halogenation)
hydrogen halides to form monohaloalkanes
water using an acid catalyst to form alcohols (hydration)
Markovnikov’s Rule
Markovnikov’s rule states that when a hydrogen halide or water is added to an unsymmetrical alkene, the hydrogen atom becomes attached to the carbon with the most hydrogen atoms attached to it already. Markovnikov’s rule can be used to predict major and minor products formed during the reaction of a hydrogen halide or water with alkenes.
Using curly arrow notation, show the reaction mechanism for the addition of a hydrogen halide to ethene
Using curly arrow notation, show the reaction mechanism for the acid catalysed addition of water to ethene
The inductive stabilisation of intermediate carbocations formed during these reactions can be used to explain the products formed
The reaction mechanism for the addition of a halogen can be represented using curly arrows and showing the cyclic ion intermediate
Carboxylic acids can be prepared by:
oxidising primary alcohols using acidified permanganate, acidified dichromate and hot copper(II) oxide
oxidising aldehydes using acidified permanganate, acidified dichromate, Fehling’s solution and Tollens’ reagent
hydrolysing nitriles, esters or amides
Reactions of carboxylic acids include:
formation of salts by reactions with metals or bases
condensation reactions with alcohols to form esters in the presence of concentrated sulfuric or concentrated phosphoric acid
reaction with amines to form alkylammonium salts that form amides when heated
reduction with lithium aluminium hydride to form primary alcohols
Amines
Organic derivatives of ammonia in which one or more hydrogen atoms of ammonia has been replaced by an alkyl group
Classification of amines
Amines can be classified as primary, secondary or tertiary according to the number of alkyl groups attached to the nitrogen atom.
Amine + acid —>
Salt
Explain why primary and secondary amines have higher boiling points than isomeric tertiary amines
Primary and secondary amines display hydrogen bonding due to the presence of the N-H bonds
Tertiary amines do not display hydrogen bonding as there are no hydrogen atoms attached directly to the nitrogen
As a result, primary and secondary amines have higher boiling points than isomeric tertiary amines
Solubility of amines
Primary, secondary and tertiary amine molecules can hydrogen-bond with water molecules, thus explaining the appreciable solubility of the shorter chain length amines in water
Although tertiary amines cannot hydrogen bonds with themselves due to the lack of N-H bond, the lone pair on the nitrogen can form hydrogen bonds with δ+ hydrogen of the water molecule
Explain how amine acts like a weak base in solution
Amines like ammonia are weak bases and dissociate to a slight extent in aqueous solution. The nitrogen atom has a lone pair of electrons which can accept a proton from water, producing hydroxide ions.
Benzene (C6H6)
Simplest member of the class of aromatic hydrocarbons
By referring to the structure of the benzene ring, explain why the benzene ring does not take part in addition reactions
The benzene ring does not take part in addition reactions due to the stability of the benzene ring
The stability of the benzene ring is due to the delocalisation of electrons in the conjugated system
Bonding in benzene
Each carbon atom can be regarded as going under a sp2 hybridisation. The overlap of the sp2 orbitals give rise to sigma bonds and the overlap of the unhybridised p orbitals give rise to pi bonds, in the case of benzene, the delocalised ring of six electrons.
Phenyl group
A benzene ring in which one hydrogen atom has been substituted by another group, –C6H5
Benzene rings can take part in electrophilic substitution reactions. Reactions at benzene rings include:
halogenation by reaction of a halogen using aluminium chloride or iron(III) chloride for chlorination and aluminium bromide or iron(III) bromide for bromination
alkylation by reaction of a haloalkane using aluminium chloride
nitration using concentrated sulfuric acid and concentrated nitric acid
sulfonation using concentrated sulfuric acid