19 - Urin: Acid-Base Equilibrium
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
What is an acidic solution?
Solution below pH of 7
What is a basic solution?
Solution above pH of 7
What is the normal pH range for blood?
7.35-7.45pH
What is physiological acidosis?
pH below 7.35
Acidic relative to normal pH range of blood
What is physiological alkalosis?
pH above 7.45
Basic relative to normal pH range of blood
What does pH measure? What kind of scale is it?
pH measures concentration of H+ ions
Negative scale, meaning that pH goes down when H+ concentration goes up (acidic = low pH, basic = high pH)
Logarithmic scale, meaning that +1 pH equal x10 H+ ions
Is physiological acidosis or alkalosis a more serious problem?
Physiological acidosis, more common and can be fatal as it can shut down CNS function
What is an equilibrium reaction?
A type of reversible chemical reaction where both products and reactants are present at the same time
Ex: Buffers
What type of reaction do all buffers participate in?
Equilibrium reactions, keeps a balance between reactants and products to keep H+ in balance
How do all buffers react to an increase in H+?
The buffer will "consume" it, forming a product between H+ and another reactant (usually)
How do all buffers react to a decrease in H+?
The buffer will produce H+, by dissociating a reactant into H+ and another product (usually)
What is the protein buffer system?
The hydrogen ions in carboxyl and amino groups on amino acids can accept or donate H+ ions depending on the pH.
If pH is low (high H+ conc.), amino acids will accept H+, thus lowering the H+ concentration
If pH is high (low H+ conc.), amino acids will donate H+, thus raising the H+ concentration
What is the equation of the bicarbonate buffer system?
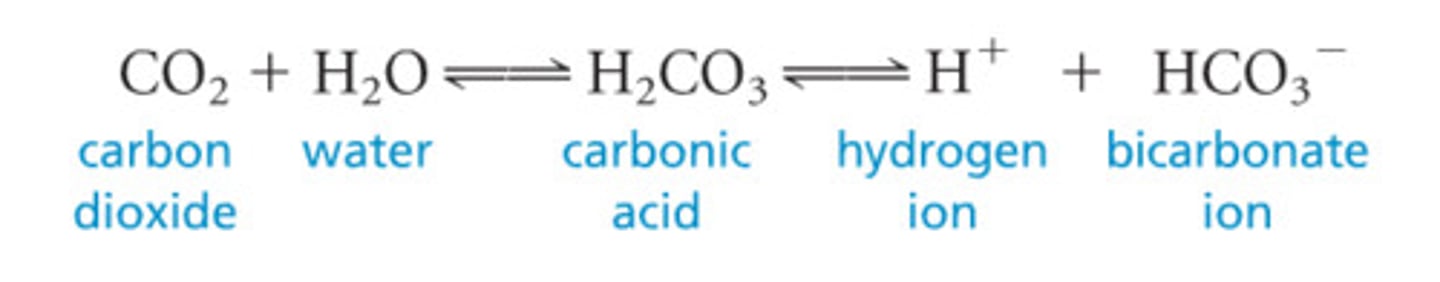
What enzyme is necessary for the bicarbonate buffer system to work?
Carbonic anhydrase
Which 2 buffer systems are present in urine and intracellular fluids?
Phosphate buffer and bicarbonate buffer system
Where does the bicarbonate buffer system operate in the body?
In intracellular and extracellular fluids
Where does the protein buffer system operate in the body?
Inside of cells and in the blood plasma
What occurs to the bicarbonate buffer system when there is excess CO2?
The reaction shifts to the right, with more hydrogen and bicarbonate ions being produced
The increased hydrogen ion concentration causes pH to drop
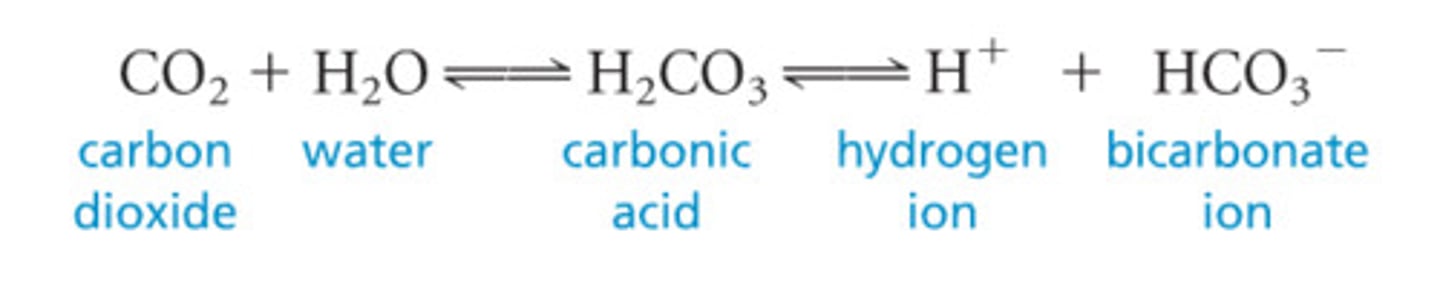
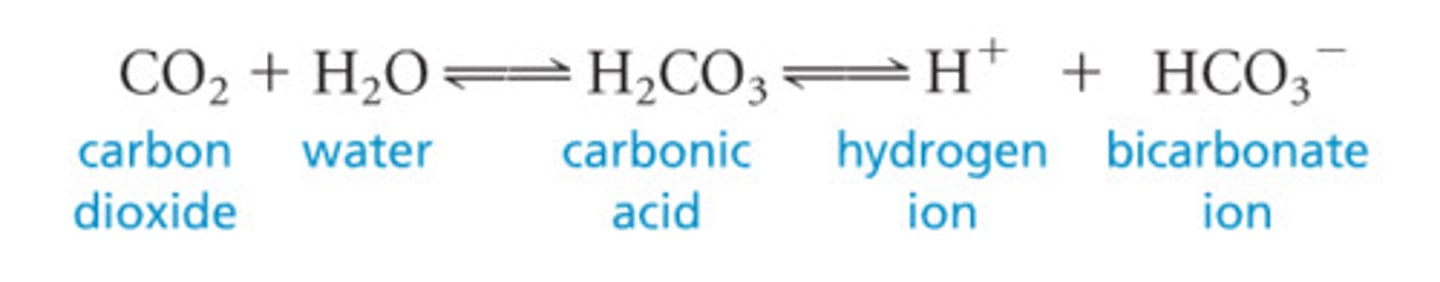
What occurs to the bicarbonate buffer system when there is excess H+?
The reaction shifts to the left, with more carbon dioxide and water (insignificant) being produced
The decreased hydrogen ion concentration causes pH to increase
What 3 conditions causes respiratory acidosis?
Anything that damages your lungs or causes you to stop breathing
-Damage to lungs, airways, or muscles of breathing
-Damage of the respiratory centers in the medulla oblongata
-Holding your breath
What hormones are released as a response to acute respiratory acidosis?
Epinephrine + norepinephrine, forces you to start breathing again
What are 6 causes of metabolic acidosis?
1. Anerobic metabolism (production of metabolic acids - lactate or lactic acid?)
2. Kidney dysfunction (unable to secrete large amounts of acids)
3. Incomplete breakdown of fatty acids (increase in fatty acids and ketone bodies)
4. Consumption of ethanol, methanol and other toxic alcohols (production of acetic acid)
5.Production of sulfuric and other acids from normal metabolism
6. Diarrhea (loss of bicarbonate)
How does hyperventilation cause respiratory alkalosis?
Causes loss of excessive amounts of co2, which results in less carbonic acid being produced, and less H+, which causes alkalosis
What happens to CO2 levels in people who "pass out" after hyperventilation?
Causes them to quit breathing for a few moments, which allows enough CO2 to be retained to stimulate breathing again
What are 3 causes of metabolic alkalosis?
1. Vomiting (excessive loss of H+ from gastric juice)
2. Ingestion of bicarbonate (consumes H+ and lowers pH)
3. Constipation (excessive absorption of bicarbonate in feces)
How are changes to physiological pH detected in the body?
Changes to blood pH is detected by chemoreceptors in the aortic arch and carotid bodies
Changes to cerebral spinal fluid pH is detected by chemoreceptors int he ventral medulla
How does respiratory compensation occur?
Acts immediately
Chemoreceptors detect changes pH in the blood and the CSF, and stimulates the respiratory centers in the medulla and the pons, which changes breathing appropriate to compensate
If pH is too acidic, more CO2 is breathed out
If pH is too basic, more CO2 is retained
How does renal compensation occur?
Acts in the long term
If pH is too acidic, more H+ ions are excreted via the Na+/H+ antiporter
If pH is too basic, more HCO3 ions are excreted
In the long term, if the pH is too acidic, more HCO3 ions can be generated via the Na+/H+ antiporter
A patient presents with a pH of 7.25, high CO2, and high bicarbonate levels.
What type of acid/base imbalance does this patient have?
How is the patient's imbalance being compensated?
Respiratory Acidosis - more CO2 is being retained, which is causing more H+ to be produced
Metabolic compensation is occurring (retention of bicarbonate so that more binds to H+ ions)
A patient presents with a pH of 7.25, low CO2, and low bicarbonate levels.
What type of acid/base imbalance does this patient have?
How is the patient's imbalance being compensated?
Metabolic Acidosis - more bicarbonate is being excreted, which causes less H+ to bind to it and is thus retained in the system
Respiratory compensation is occurring (excretion of CO2 to produce less H+ ions)
A patient presents with a pH of 7.35, low CO2, and low bicarbonate levels.
What type of acid/base imbalance does this patient have?
How is the patient's imbalance being compensated?
Respiratory alkalosis - not enough CO2 is being retained, which causes less H+ to be produced
Metabolic compensation is occurring (retention of bicarbonate - causes less H+ to bind to it and thus retained)
A patient presents with a pH of 7.35, high CO2, and high bicarbonate levels.
What type of acid/base imbalance does this patient have?
How is the patient's imbalance being compensated?
Metabolic alkalosis - too much bicarbonate is being retained, which causes more H+ to bind to it, and thus less is retained in the system
Respiratory compensation is occurring (retention of CO2 to produce more H+ ions)