Reactions of Complex Ions in Aqueous Solutions
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
what are metal aqua ions
Metal aqua ions are complex ions that only contain water ligands.
acidity of metal aqua solutions in solution
Metal aqua ions are acidic in solution.
what is the acidity of metal aqua ion depend on
The acidity of metal aqua ions depends on the charge density of the metal ion.
[Fe(H2O)6]3+ is more acidic than [Fe(H2O)6]2+
Fe(II) metal aqua ion molecular equation and colour in aqueous ion
[Fe(H2O6]2+ (aq)
Green s0lution
Cu metal aqua ion molecular equation and colour in aqueous ion
[Cu(H2O)6]2+ (aq)
Blue solution
Fe(III) metal aqua ion molecular equation and colour in aqueous ion
[Fe(H2O)6] yellow solution
Aluminium(III) metal aqua ion molecular equation and colour in aqueous ion
[Al(H2O)6]3+ (aq)
Colourless solution
Iron (II) +NaOH molecular equation and colour
Fe(OH)2(H2O)4 (s)
Green precipitate (grows brown in air)
Copper (II) + NaOH molecular equation and colour
CU(OH)2(H2O)4
Blue perciptate
Iron (III) + NaOH molecular equation and colour
Fe(OH)3(H2O)3
Brown precipitate
Aluminium (III) + NaOH (aq) molecular equation and colour
Al(OH)3(H2O)3
White precipitate
Iron (II) + excess NaOH(aq) molecular equation and colour
No further change
Copper (II) + excess NaOH(aq) molecular equation and colour
No further change
Iron (III) + excess NaOH(aq) molecular equation and colour
No further change
Aluminium (III) + excess NaOH(aq) molecular equation and colour
Al(OH)3(H2O)3
white precipitate
Special feature about Aluminum Hydroxide
it reacts with both acids and bases, we call it amphoteric.
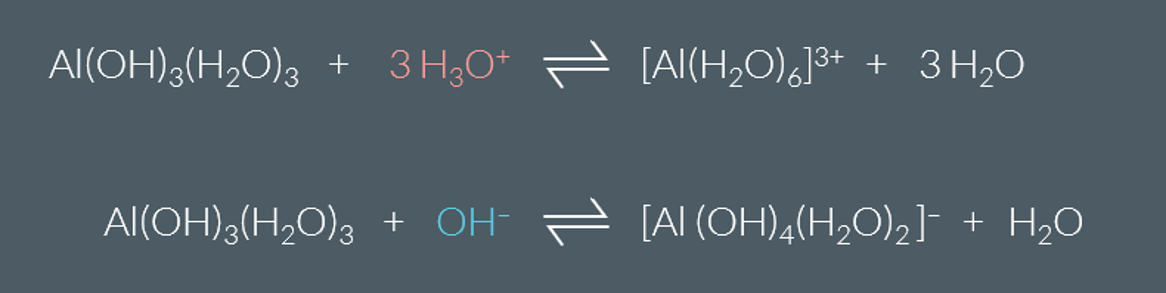
Iron (II) + NH3(aq) molecular equation and colour
Fe(OH)2(H2O)4 (s)
Green precipitate (goes brown in air)
Copper(II)+ NH3(aq) molecular equation and colour
CU(OH)2(H2O)4
Blue precipitate
Iron(II) + NH3(aq) molecular equation and colour
Fe(OH)3(H2O)3
Brown precipitate
Aluminium (III) + NH3(aq) molecular equation and colour
Al(OH)3(H2O)3
White precipitate
Iron (II) + excess NH3 (aq) molecular equation and colour
no further change
Copper (II) + excess NH3 (aq) molecular equation and colour
[Cu(NH3)4(H2O)2]2+ (aq)
Deep blue solution
Iron (III)+ excess NH3 (aq) molecular equation and colour
No further change
Aluminium (III) + excess NH3 (aq) + excess NH3 (aq) molecular equation and colour
No further change
Iron (II) + Na2CO3 (aq) molecular equation and colour
FeCO3 (s)
Green precipitate
Copper (II) + Na2CO3 (aq) molecular equation and colour
CuCO3 (s)
Blue-Green precipitate
Iron (III) + Na2CO3 (aq) molecular equation and colour
Fe(OH)3(H2O)3
Brown precipitate
bubbles of CO3
Aluminium (III) + Na2CO3 (aq) molecular equation and colou
Al(OH)3(H2O)3
White percipitate
Bubbles of CO2