Microbiology - Section 1, Lesson 9
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
Q: What are the two basic types of specimen preparation for light microscopy?
A: Wet mounts and fixed specimens.
Q: What is a wet mount?
A: A preparation where a specimen is placed in a drop of liquid on a slide, often with a coverslip on top.
Q: What types of specimens can be used for wet mounts?
A: Liquid samples (like urine) or solids (like skin scrapings) mixed with a drop of liquid.
Q: Why are stains sometimes added to wet mounts?
A: To enhance contrast and make features of the specimen easier to see.
Q: What is fixation in microscopy?
A: The process of attaching cells to a slide, which also kills the microorganisms and preserves cell structure.
Q: What are the two main methods of fixation?
A: Heat fixation and chemical fixation.
Q: How is heat fixation performed?
A: A thin smear of the specimen is placed on a slide and then briefly heated over a heat source.
Q: What is a smear in microscopy?
A: A thin layer of a specimen spread on a microscope slide for fixation and staining.
Q: Why is chemical fixation sometimes preferred over heat fixation?
A: It’s often better for tissue samples because it more effectively preserves structure without damaging the specimen.
Q: Name some common chemical fixatives used in microscopy.
A: Acetic acid, ethanol, methanol, formaldehyde (formalin), and glutaraldehyde.
Q: What do chemical fixatives do to biological specimens?
A: They denature proteins, stop biochemical reactions, and stabilize cell and tissue structures.
Q: What tool can be used to heat-fix a specimen besides a flame?
A: A slide warmer.
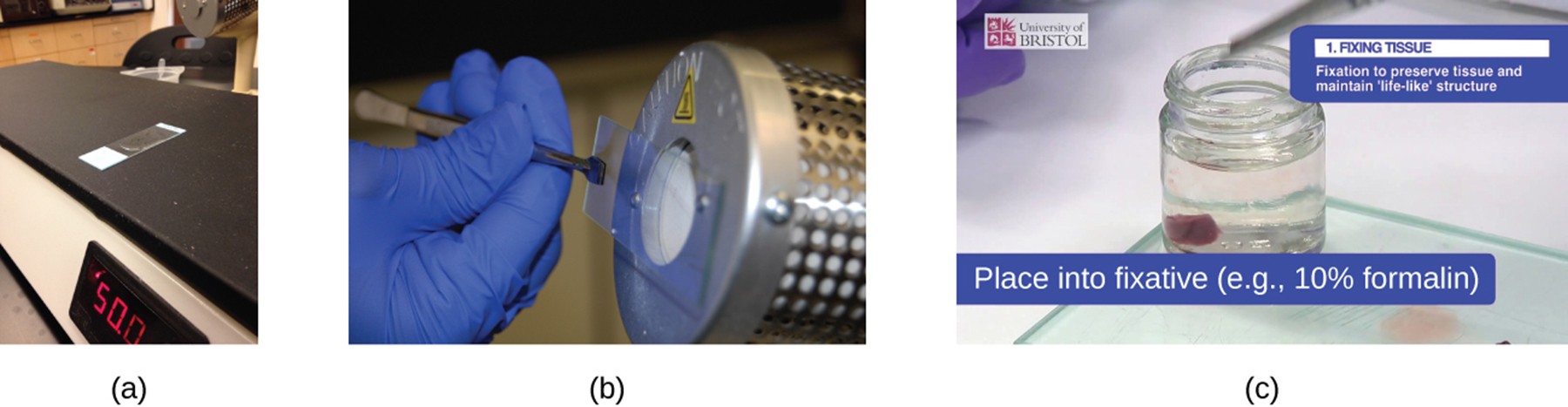
Q: What is another common method for heat-fixing a slide besides a slide warmer?
A: Holding the slide with a smear over a microincinerator.
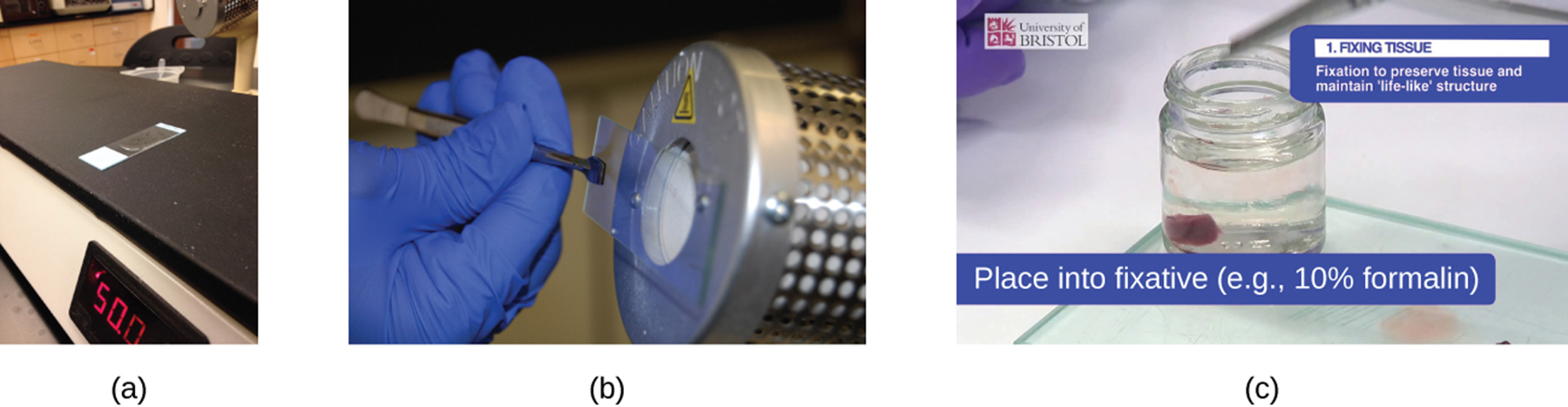
Q: How is chemical fixation of tissues commonly performed?
A: By placing the tissue sample in a solution of formalin (formaldehyde).
Q: What is the main purpose of chemical fixation in microscopy?
A: To kill microorganisms, stop tissue degradation, and preserve structure for observation.
Q: Why is staining applied to specimens before examining them under a light microscope?
A: To color certain features of the specimen, enhancing contrast and making structures easier to see.
Q: What are stains (or dyes) chemically composed of?
A: Salts made up of a positive ion and a negative ion.
Q: What is a chromophore in a stain?
A: The colored ion (either positive or negative) responsible for the dye’s color.
Q: What is a counterion in a stain?
A: The uncolored ion paired with the chromophore.
Q: How is a stain classified if the chromophore is the positively charged ion?
A: It is called a basic dye.
Q: How is a stain classified if the chromophore is the negatively charged ion?
A: It is called an acidic dye.
Q: What determines which dye is selected for staining a specimen?
A: The chemical properties of both the dye and the specimen, which influence how they interact.
Q: What is a positive stain?
A: A dye that is absorbed by the cells or organisms, coloring the objects of interest against a clear background.
Q: What is a negative stain?
A: A dye that stains the background but not the cells or organisms, producing an outline or silhouette of the specimen.
Q: Why might negative staining be advantageous?
A: It highlights the shape and outline of organisms by contrasting them against a colored background.
Q: What stain did Bacillus anthracis cells absorb in the example?
A: Crystal violet, a basic positive stain.
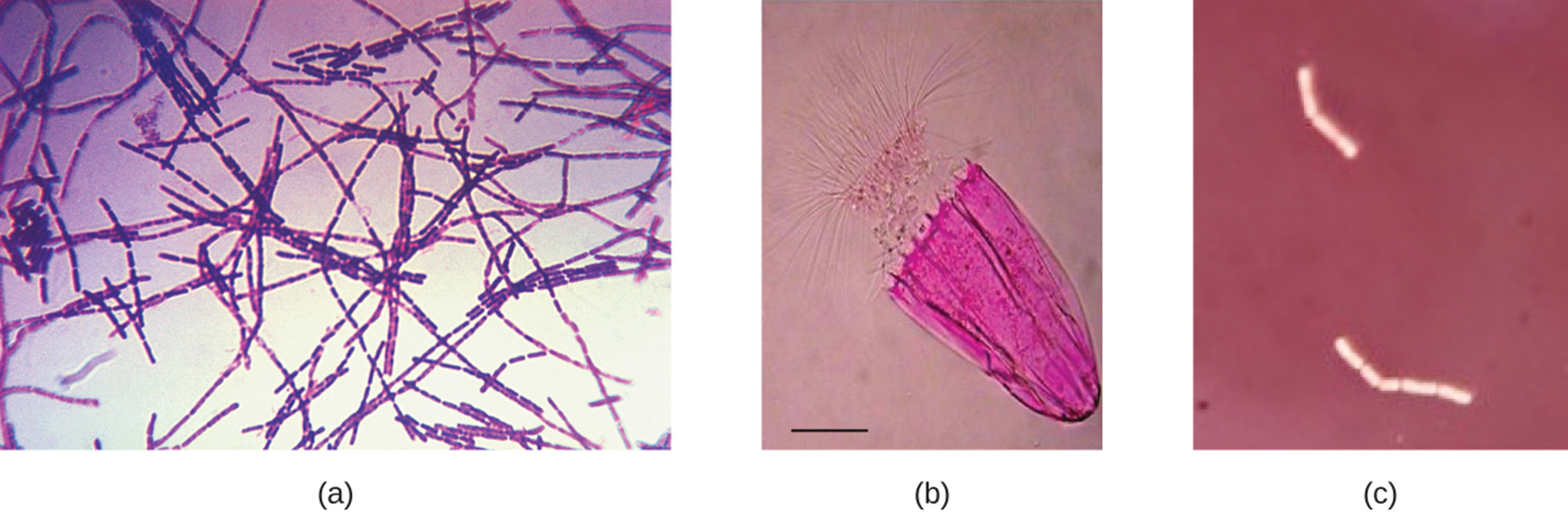
Q: What type of stain is rose bengal, used on the Spinoloricus specimen?
A: A positive acidic stain.
Q: Why do Bacillus megaterium cells appear white when a negative red stain is applied?
A: Because they do not absorb the negative stain, so only the background is stained.
Q: What does it mean if a cell does not absorb a stain in negative staining?
A: The cell appears unstained (white) and stands out as a silhouette against a colored background.
Q: Why do basic dyes tend to stick to cell walls and act as positive stains?
A: Because cells have negatively charged cell walls, and the positive chromophores in basic dyes are attracted to them.
Q: Name some commonly used basic dyes that serve as positive stains.
A: Basic fuchsin, crystal violet, malachite green, methylene blue, and safranin.
Q: Why do acidic dyes act as negative stains?
A: Because their negatively charged chromophores are repelled by negatively charged cell walls and stain the background instead.
Q: Name some commonly used acidic dyes.
A: Acid fuchsin, eosin, and rose bengal.
Q: What is simple staining?
A: The use of a single dye to color all organisms similarly, emphasizing structures but not differentiating between species.
Q: What is differential staining?
A: Using multiple stains to distinguish between organisms or structures, making them appear different colors.
Q: Name some common differential staining techniques used in clinical microbiology.
A: Gram staining, acid-fast staining, endospore staining, flagella staining, and capsule staining.
Q: What is a wet mount?
A: A slide preparation technique in which a specimen is placed on the slide in a drop of liquid.
Q: What is fixation?
A: The process by which cells are killed and attached to a slide.
Q: What is a smear?
A: A thin layer of a specimen spread on a slide.
Q: What is staining in microscopy?
A: The addition of stains or dyes to a microscopic specimen to enhance contrast.
Q: What is a basic dye?
A: A chromophore with a positive charge that attaches to negatively charged structures.
Q: What is an acidic dye?
A: A chromophore with a negative charge that attaches to positively charged structures.
Q: What is a positive stain?
A: A stain that colors the structure of interest.
Q: What is a negative stain?
A: A stain that colors the background around the structure but not the structure itself.
Q: What is simple staining?
A: A staining technique that uses a single dye.
Q: What is differential staining?
A: Staining that uses multiple dyes to differentiate between structures or organisms.
Q: What type of staining procedure is the Gram stain?
A: A differential staining procedure.
Q: Who developed the Gram stain and in what year?
A: Hans Christian Gram in 1884.
Q: What is the purpose of the Gram stain?
A: To distinguish bacteria based on differences in their cell wall structure, especially peptidoglycan thickness.
Q: What is the first step of the Gram stain procedure?
A: Apply crystal violet, the primary stain, which gives all cells a purple color.
Q: What is the role of Gram’s iodine in the Gram stain?
A: It acts as a mordant, forming a crystal violet–iodine complex that gets trapped in thick peptidoglycan layers.
Q: What is a mordant?
A: A substance that sets or stabilizes a stain, often by forming a complex with the dye.
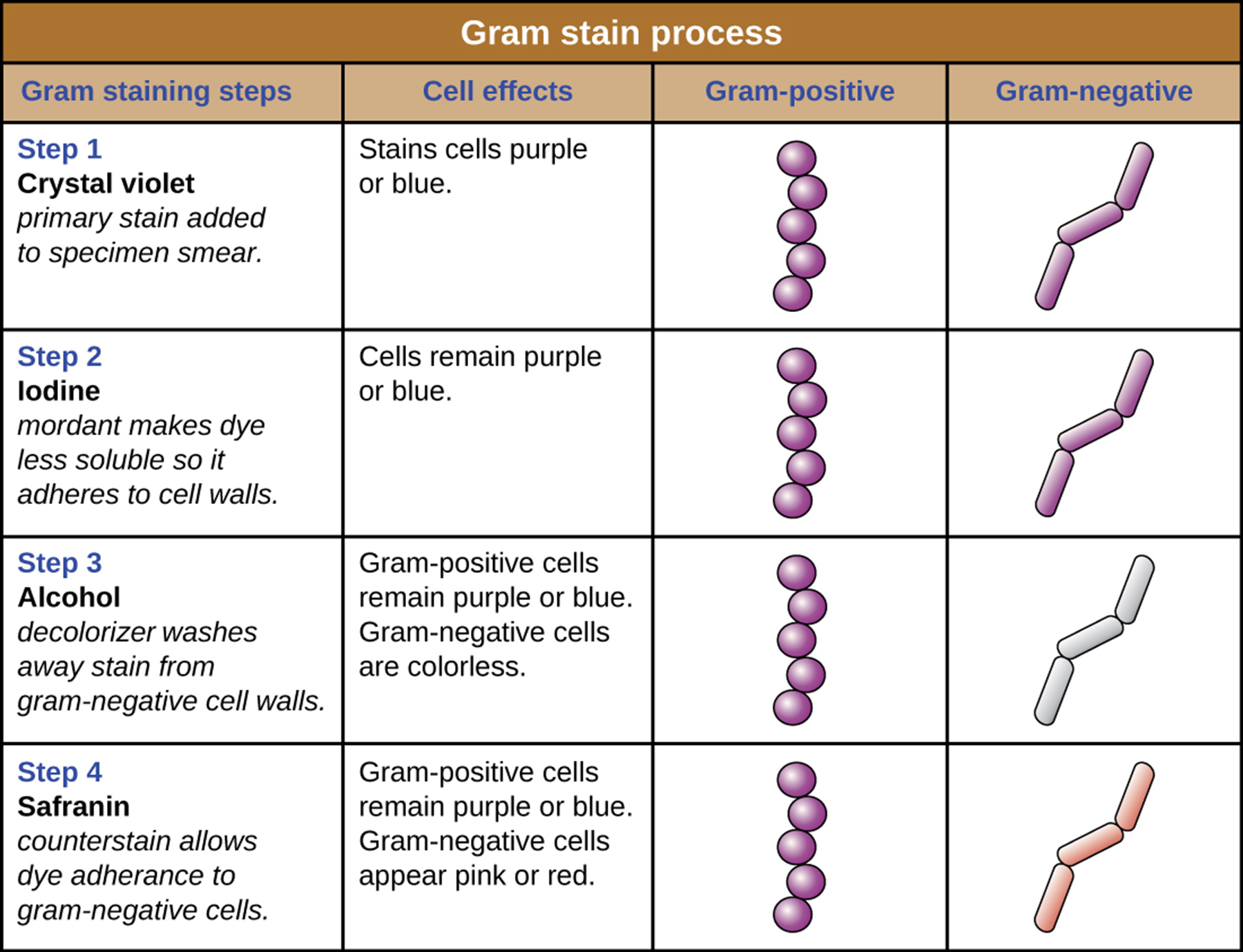
Q: What is the third step of the Gram stain and its effect?
A: Apply a decolorizing agent (ethanol or acetone/ethanol); it removes the stain from cells with thin peptidoglycan layers, making them colorless.
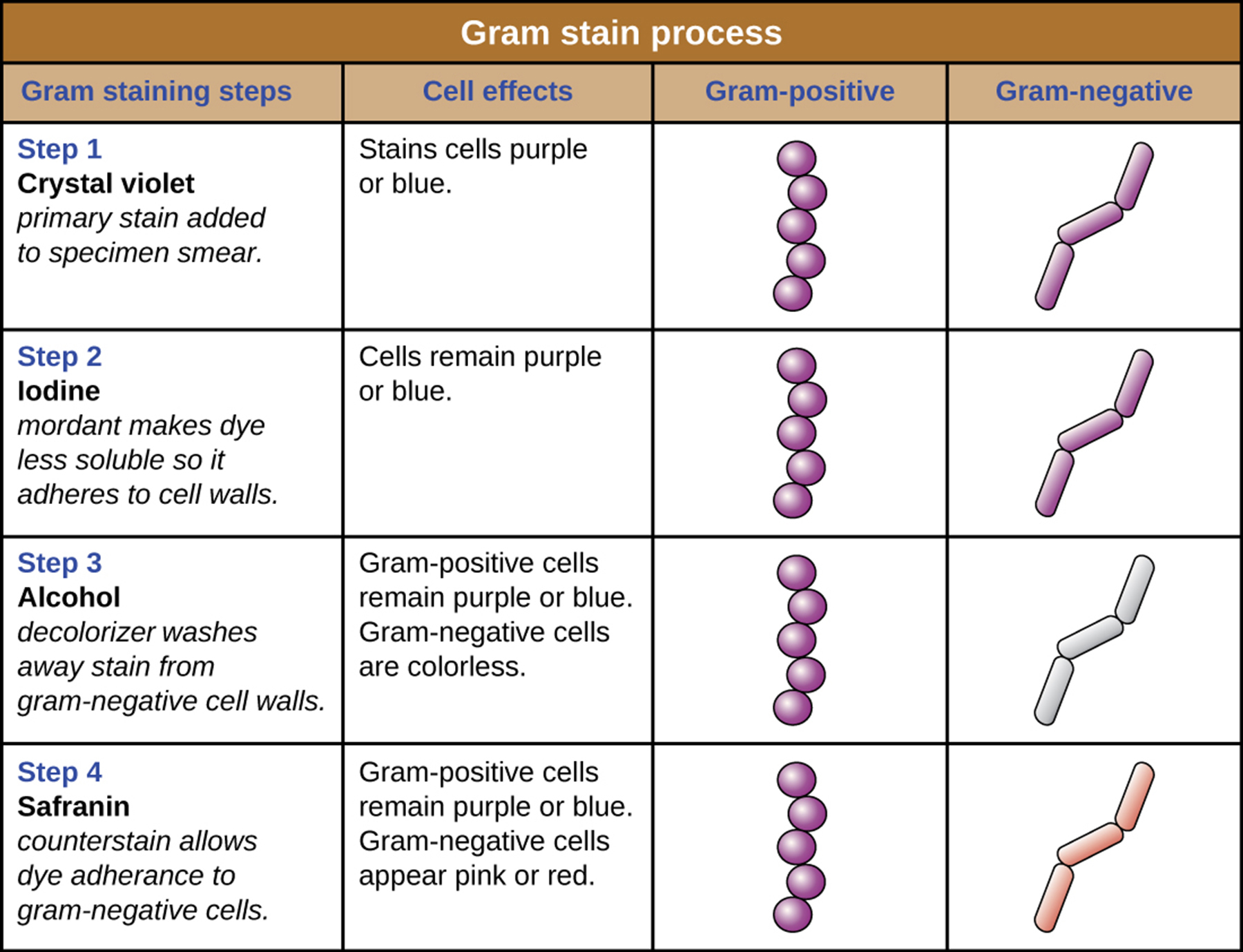
Q: Which type of cells retain the crystal violet–iodine complex and remain purple?
A: Cells with thick peptidoglycan layers (Gram-positive).
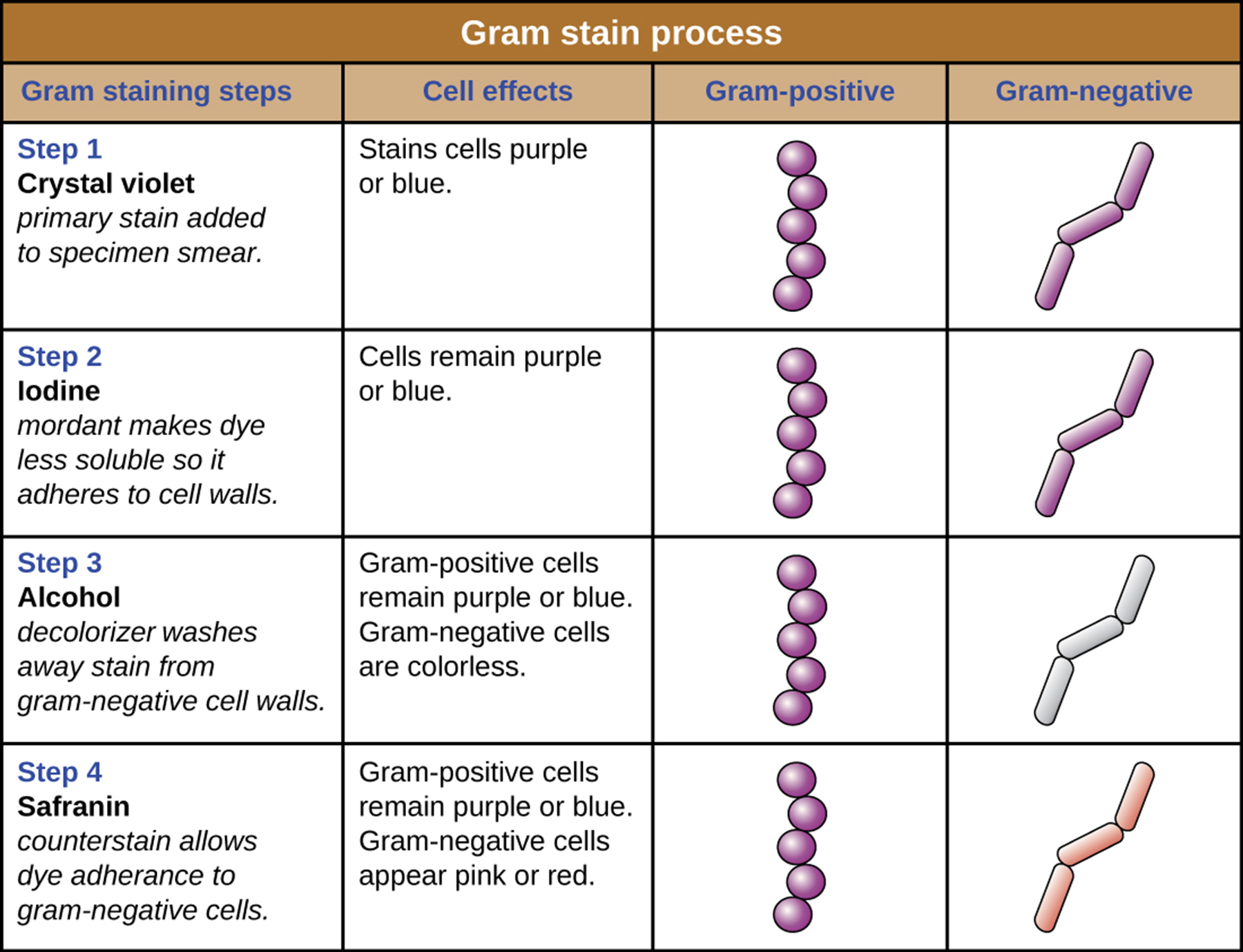
Q: What is the final step of the Gram stain?
A: Add safranin, a secondary counterstain, which stains the decolorized cells pink.
Q: What color are Gram-positive cells after the Gram stain is completed?
A: Purple
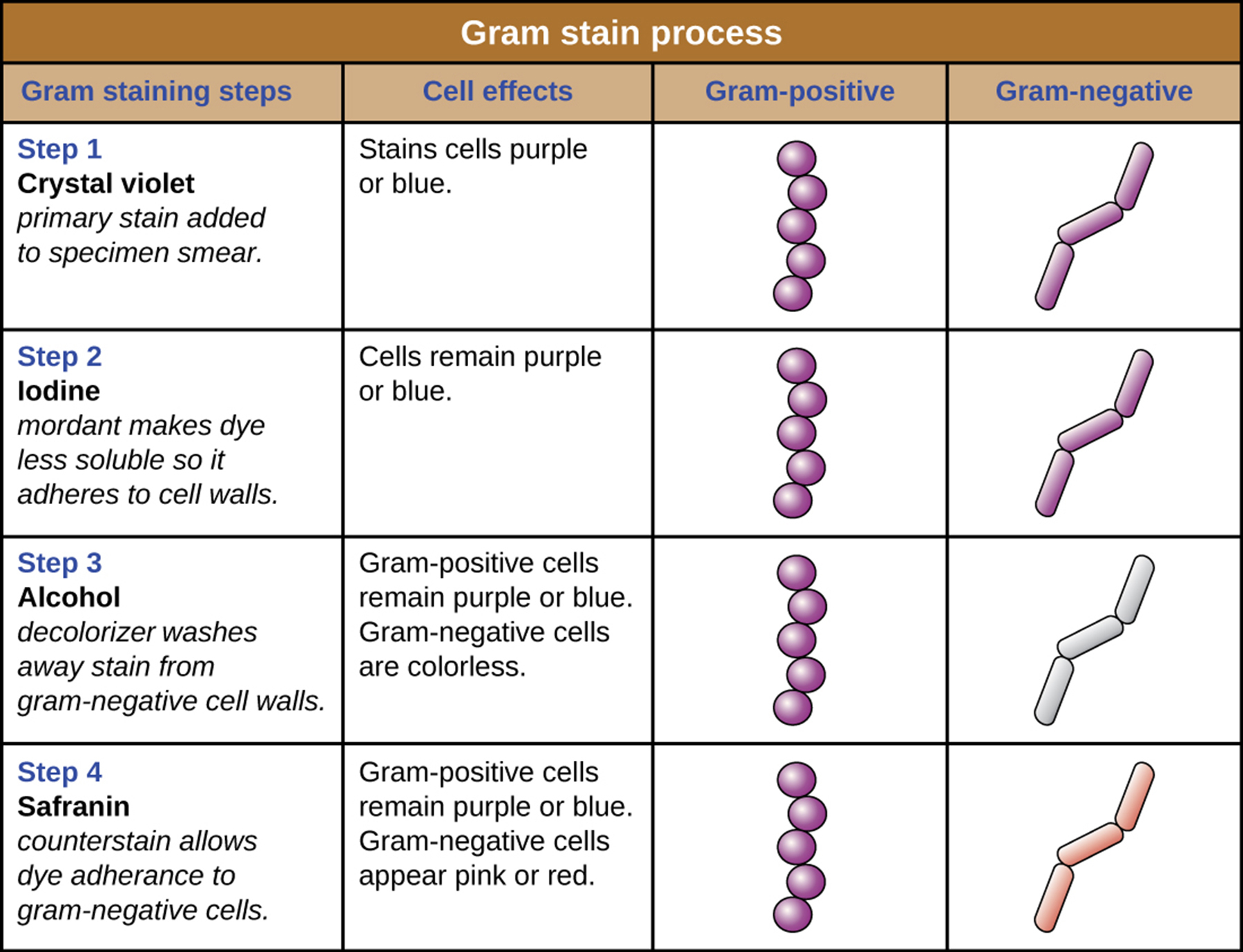
Q: What color are Gram-negative cells after the Gram stain is completed?
A: Pink
Q: What color do gram-positive cells appear after a Gram stain?
A: Purple (from crystal violet).
Q: What color do gram-negative cells appear after a Gram stain?
A: Red or pink (from safranin).
Q: What is one reason a gram-positive species might appear gram-negative?
A: Old bacterial cells may have damaged cell walls, causing loss of crystal violet stain.
Q: Why should fresh bacterial cultures be used for Gram staining?
A: Because older cells may lose stain and give false gram-negative results.
Q: What staining error can cause false results in Gram staining?
A: Leaving the decolorizer on too long, which may cause over-decolorization and false gram-negative appearance.
Q: If most cells in a Gram stain are purple and some are pink, what should be considered?
A: It may be due to cell damage or over-decolorization; the species may still be gram-positive if it's a pure culture.
Q: Why is Gram staining clinically important?
A: It helps classify pathogens and predict characteristics like antibiotic resistance.
Q: Which type of bacteria is generally more resistant to antibiotics—gram-positive or gram-negative?
A: Gram-negative bacteria.
Q: What is a primary stain in differential staining techniques?
A: The first dye added to the specimen.
Q: What is the function of a mordant in staining?
A: A chemical that helps set or stabilize a stain in the specimen.
Q: What does a decolorizing agent do in staining procedures?
A: It removes the primary stain, usually from certain parts of the specimen.
Q: What is a counterstain?
A: A secondary dye that provides contrast by coloring cells that lost the primary stain.
Q: What is the purpose of acid-fast staining?
A: To differentiate gram-positive cells with waxy mycolic acids in their cell walls from those without.
Q: What is the primary stain used in both acid-fast techniques?
A: Carbolfuchsin.
Q: What type of cells retain carbolfuchsin even after acid-alcohol decolorization?
A: Acid-fast cells.
Q: What is the counterstain used in acid-fast staining?
A: Methylene blue.
Q: What color do acid-fast bacteria appear after staining?
A: Red or pink.
Q: What color do non–acid-fast bacteria appear after staining?
A: Blue.
Q: What is the main difference between the Ziehl-Neelsen and Kinyoun acid-fast staining methods?
A: Ziehl-Neelsen uses heat during staining; Kinyoun does not.
Q: Why is acid-fast staining clinically important?
A: It helps diagnose diseases caused by acid-fast bacteria, like tuberculosis.
Q: How is Mycobacterium tuberculosis typically detected under a microscope?
A: With acid-fast staining of a sputum smear using the Ziehl-Neelsen technique.
Q: What alternative method can be used to detect M. tuberculosis using fluorescence?
A: Immunofluorescence with fluorochrome-labeled antibodies.
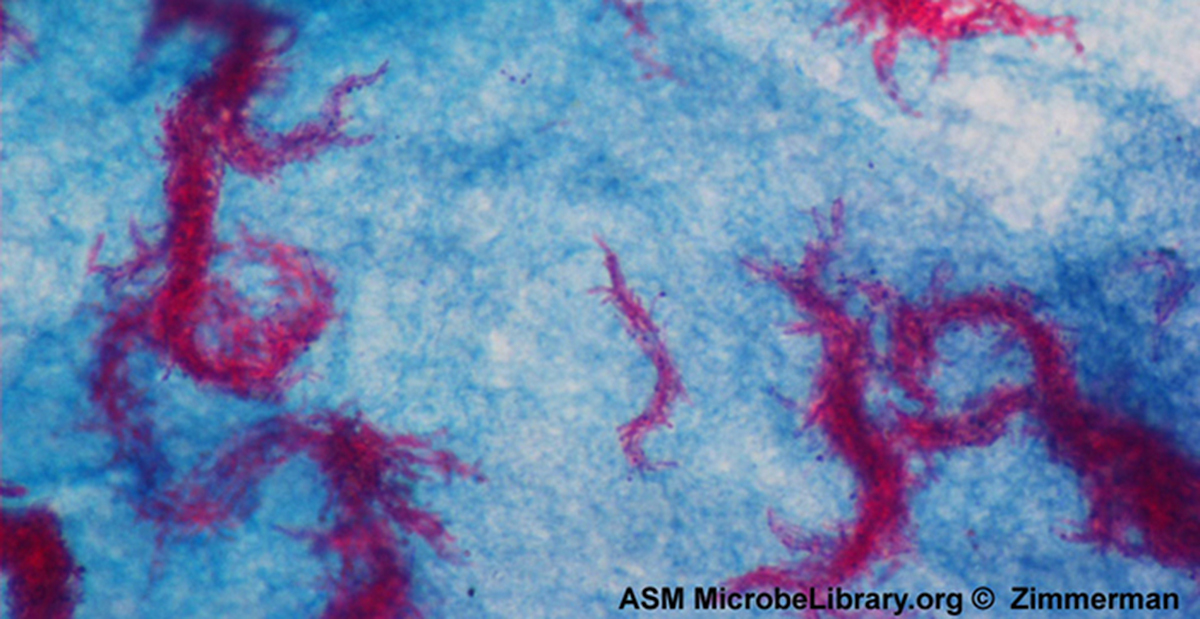
Q: What is a capsule in microbiology?
A: A protective outer structure found in certain bacteria and yeasts.
Q: Why is capsule detection important clinically?
]]
A: Because capsules increase a microbe’s virulence, or ability to cause disease.
Q: Why are capsules typically visualized with negative staining?
A: Capsules do not absorb most basic dyes, so negative staining stains the background instead.
Q: What do capsules look like after negative staining?
A: They appear as halos around the stained cells.
Q: Is heat fixation required for capsule staining?
A: No, heat fixation is not used, as it could distort or destroy the capsule.
Q: What two dyes are commonly used for negative capsule staining?
A: India ink and nigrosin.
Q: How can both positive and negative stains be combined in capsule staining?
A: The cell body is stained with a positive stain, and the background with a negative stain, leaving a clear halo for the capsule.
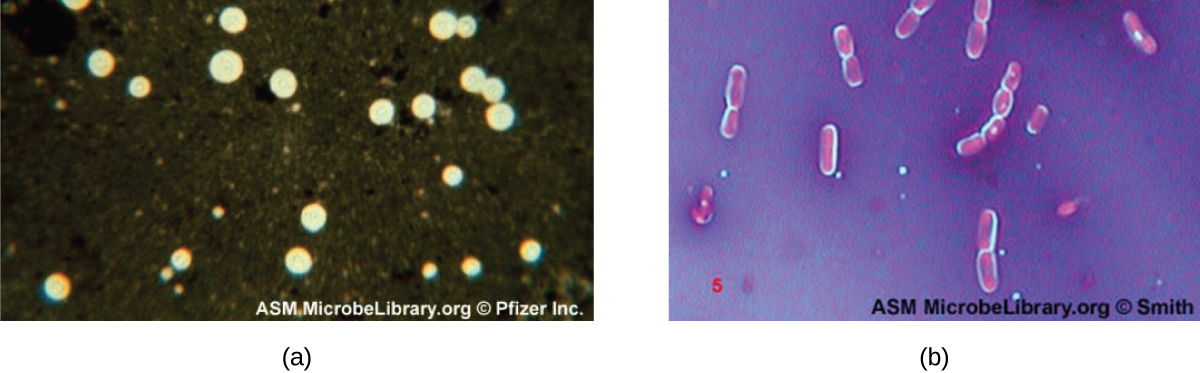
Q: What organism is shown with halos using India ink in capsule staining?
A: Cryptococcus neoformans, a yeast with polysaccharide capsules.
Q: What causes the halo effect when using India ink with Cryptococcus neoformans?
A: The capsule does not absorb the dye, leaving a clear halo around the cell.
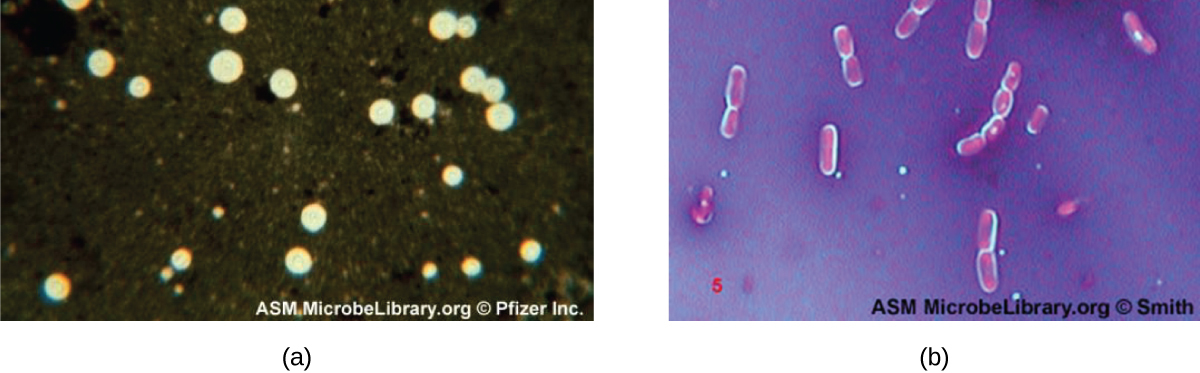
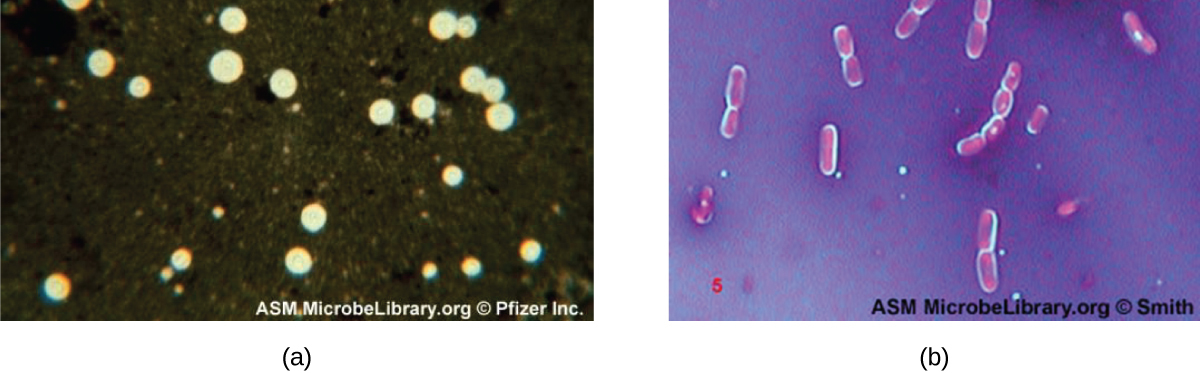
Q: Why do Bacillus cells show halos when stained with crystal violet and copper sulfate?
A: These dyes cannot penetrate the capsule, creating a light-blue halo around encapsulated cells.
Q: What color halo is seen around encapsulated Bacillus cells in a negative stain using crystal violet and copper sulfate?
A: Light-blue halo.
Q: What are endospores?
A: Structures formed inside certain bacterial cells that allow survival in harsh conditions.
Q: Why can’t Gram staining alone visualize endospores?
A: Endospores appear clear when Gram-stained because they do not absorb the stain.
Q: What is the most commonly used method for endospore staining?
A: The Schaeffer-Fulton method.
Q: What primary stain is used in the Schaeffer-Fulton method?
A: Malachite green, driven into the endospore with heat.
Q: What counterstain is used in the Schaeffer-Fulton endospore stain?
A: Safranin, which stains the vegetative cell pink.
Q: What color do endospores appear after Schaeffer-Fulton staining?
A: Green, either within or outside pink vegetative cells.
Q: What two genera are commonly identified using endospore staining?
A: Bacillus and Clostridium.
Q: Why is Bacillus anthracis significant in endospore studies?
A: It causes anthrax and is a potential bioterrorism agent.
Q: What infection is associated with Clostridium difficile?
A: C. diff, a common hospital-acquired infection.
Q: What does an acid-fast stain differentiate?
A: Cells that have waxy mycolic acids in their gram-positive cell walls.