IB HL Chemistry (first exams 2025) - Structure 2.1, 2.2, 2.3, 2.4
1/100
Earn XP
Description and Tags
2.1 Ionic Model 2.2 Covalent Model 2.3 Metallic Model 2.4 From Models to Materials
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
101 Terms
ions
charged particle
cation
positive ion formed by metals when they lose one or more electrons
anion
negative ion formed by non-metals when they gain one or more electron
writing ionic charge
number before sign (1+)
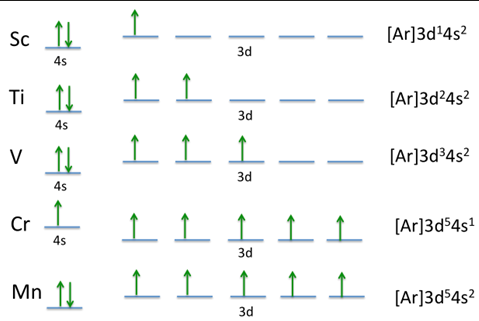
transition elements
elements that have electron configurations which allow them to lose different amounts of electrons, so can form more than one stable ion
polyatomic ions
a covalently bonded set of two or more atoms, or of a metal complex can be considered to behave as a single unit
NH4+
ammonium
NO3-
nitrate
OH-
hydroxide
HCO3-
hydrogen carbonate
CO32-
carbonate
SO42+
sulfate
PO43-
phosphate
ionic compounds
electrostatic attractions between a metal and non-metal
ionic compound properties
high brittleness due to ions of like charge near each other, high melting/boiling points due to strong electrostatic attractions, low volatility due to strong electrostatic attractions, molten/dissolved conduct electricity
lattice enthalpy
the energy needed to convert one mole of ionic solid into gaseous ions infinitely far apart under standard conditions (breaking apart), endothermic
size of lattice enthalpy
controlled by the charges on the ions, their ionic radii, and the packing arrangement of the ions
covalent bonds
two non-metals reacting together to achieve a stable configuration, where a shared pair of electrons is concentrated in the region between the two positively charged nuclei
octet rule
the tendency of covalent bonds to form a stable arrangement of eight electrons in the outer shell
octet rule exceptions
hydrogen only has two, Be and B have less than eight, S and P can have expanded octets (more than eight)
coordinate covalent bonds
form when both electrons in a shared pair originate from the same atom (donated to the other)
single bonds
share one pair of electrons, weakest and longest bond
bond enthalpy
a measure of the energy required to break the bond
double bonds
stronger and shorter than single bonds, longer and weaker than triple bonds
triple bonds
strongest and shortest bond
dipole
a pair of separated equal and opposite electrical charges located on a pair of atoms within a molecule
dipole moment
the product of the charge on a dipole and the distance between the ends; a measure of the polarity of a bond
non-polar molecule
a molecule that has a symmetric distribution of charge and whose individual bond dipoles sum to zero or cancel
polar molecule
a molecule that has an asymmetric distribution of charge: the individual bond dipoles do not sum to zero or cancel
0 - 0.4
pauling scale non-polar covalent
0.4 - 1.8
pauling scale polar covalent
> 1.8
pauling scale ionic
molecular polarity
depends on the polar bonds a molecule contains, and the shape
valence shell electron pair repulsion model
VSEPR model
VSEPR
states that in a small molecule, the pairs of valence electrons are arranged as far apart from each other possible (lone pairs and bonded pairs)
lone pair - lone pair
greatest VSEPR repulsion
lone pair - bonded pair
median VSEPR repulsion
bond pair - bond pair
weakest VSEPR repulsion
linear bonds
two atoms bonded to the central atom, no lone pairs on the central atom, 180 degree bond angle
bent
two atoms bonded to the central atom with one or two lone pairs of electrons on the central atom, 104.5 degrees bond angle
trigonal planar
three atoms bonded to the central atom, no lone pairs of electrons on the central atom, 120 degrees bond angle
trigonal pyramidal
the atoms bonded to the central atom, one lone pair of electrons on the central atom, 107 degrees bond angle
tetrahedral
four atoms bonded to the central atom, no lone pairs of electrons on the central atom, 109.5 degrees bond angle
linear - linear
180, 2 bonding pairs, 0 lone pairs
trigonal planar - trigonal planar
120, 3 bonding pairs, 0 lone pairs
trigonal planar - bent linear
118, 2 bonding pairs, 1 lone pair
tetrahedral - tetrahedral
109.5, 4 bonding pairs, 0 lone pairs
tetrahedral - trigonal pyramid
107, 3 bonding pairs, 1 lone pair
tetrahedral - bent linear
104.5, 2 bonding pairs, 2 lone pairs
octahedral
6 electron domains, angles of 90 degrees and d2sp3 hybridisation
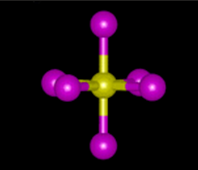
square pyramidal
derivative of the octahedral with one lone pair of electrons on the top or bottom, 90 degrees bond angle
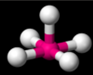
square planar
derivative of the octahedral shape with two lone pairs of electrons, one on top and one on the bottom, 90 degrees bond angle
intermolecular forces
forces between molecules, london/van der waals/dispersion forces, dipole-dipole forces, hydrogen bonding
intramolecular forces
forces within the molecule such as covalent, ionic, and metallic bonding
london/van der waals/dispersion forces
IMF in all covalents, only IMF in non-polar molecules
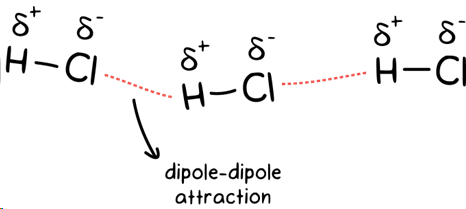
dipole - dipole forces
IMF only present in polar molecules with permanently charged regions, stronger than dispersion forces, and strength depends on degree of polarity, caused when molecules with permanent dipoles attract each other
hydrogen bonding
strongest IMF, type of dipole - dipole attraction, form when hydrogen bonds to oxygen, nitrogen, or fluorine, strength due to hydrogen small size and large electronegativity of N, O, and Fj
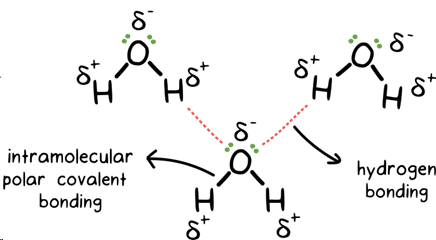
like dissolves like
polar/ionic solvents dissolve polar/ionic solutes, non-polar solvents dissolve non-polar solutes
higher
stronger IMF, ________ boiling point
lower
stronger IMF, ________ volatility
dispersion > dipole-dipole > hydrogen bonding
order of volatility in covalent IMFs
dispersion < dipole-dipole < hydrogen bonding
order of melting/boiling point in covalent IMFs
covalents electrical conductivity
generally do not conduct, some giant covalents, some polar covalents
graphite, graphene, diamond, fullerene
allotropes of carbon
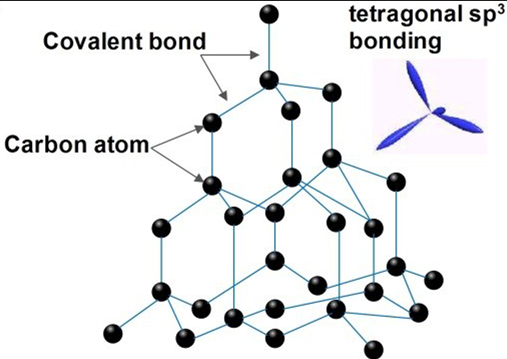
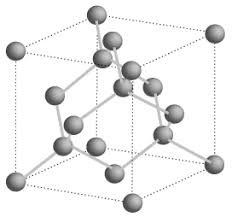
diamond
each C atom is sp3 hybridised covalently bonded to 4 others tetrahedrally in a repeating pattern with bond angles of 109.5, density is 3.51 g/cm3, all electrons bonded so no conductivity, high melting point, brittle
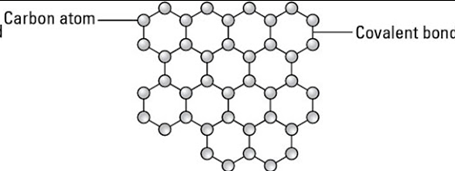
graphene
c atom is sp3 hybridized and covalently bonded to three other carbons forming hexagons with bond angles 120, two dimensional single layer in a hexagonal pattern, density 1.5g/cm3, one delocalised electron per atom so conducts electricity, excellent heat conductor, high melting point
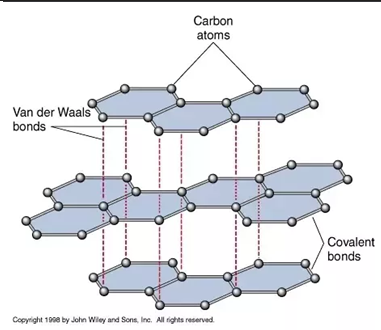
graphite
parallel layers of graphene, held together by van der waals (dispersion) forces so they can slide over each other, density is 2.26g/cm3, not a good heat conductor, high melting point, most stable allotrope
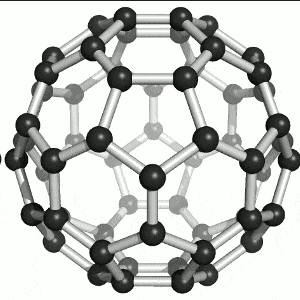
fullerene
c atom is sp2 hybridised and covalently bonded in a sphere, closed spherical cage, density is 1.726g/cm3, easily accepts electrons so is a semiconductor, low heat conductivity, low melting point, soluble in benzene
Silicon
group four element with four valence electrons, each Si atom is covalent bonded to four others in a tetrahedral arrangement, results in giant lattice structure
silica/quartz (SiO2)
tetrahedral structure with bonds between Si and O, each Si bonded to 4 oxygen atoms and each O bonded to two Si, strong, insoluble in water, does not conduct electricity or heat, high melting point
formal charge = (no. valence electrons) - ½ (no. bonding electrons) - (no. non-bonding electrons)
formal charge formula extended
FC = V - 1/2B - N
formal charge formula shortened
formal charge
the charge assigned to an atom in a molecule
preferred lewis structure
where the difference on formal charge is closest to zero, the negative charges located on the most electronegative atomd
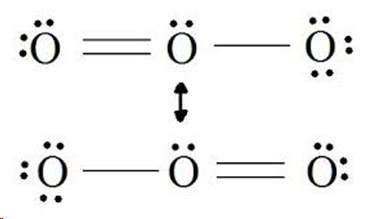
resonance
a concept used to describe the structures when there are multiple ways to depict the same molecule
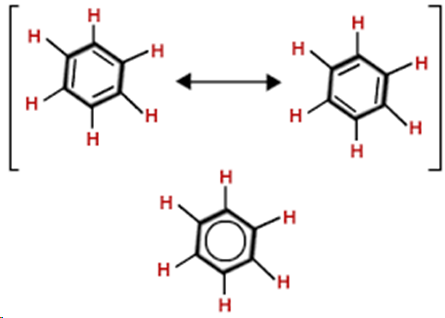
benzene C6H6
colourless compound, physical state is liquid with slight aromatic smell, less dense than H2O, immiscible with H2O, miscible with non-polar solvents
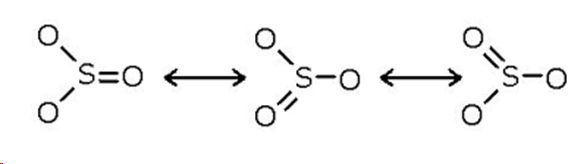
ozone O3
bent shape with bond angle of 117 degrees, two resonance structures, double bond with one pi and one sigma bond, bond order is 1.5 which means the length is intermediate and the strength is between a double and single bond
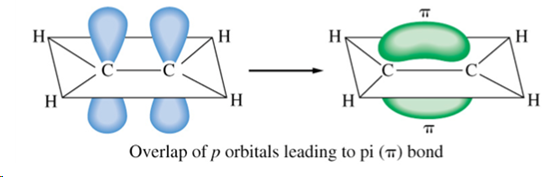
pi bonds
form by the lateral combination of p-orbitals, where the electron density is concentrated on opposite sides of the bond axis (so weaker than sigma), found in double and triple bonds
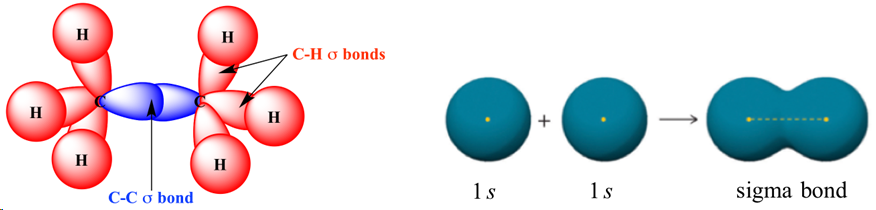
sigma bonds
strongest type of covalent chemical bond, formed by head on overlapping between atomic orbitals along the bond axis, all single covalent bonds, formed by s/s, s/p, p/p, hybrid/s, hybrid/hybrid
hybridisation
model that describes the changes on the atomic orbitals of an atom when it forms a covalent compound (sp, sp2, sp3)
reasons for hybridisation
VSEPR theory requires molecules to have identical orbitals at the stage of bonding, so the s and p orbitals hybridise to be identical
hybrid orbitals
only found in covalent compounds, equivalent in a compound, no. is equal to the no. of atomic orbitals used to form it, type depends on electron domain geometry
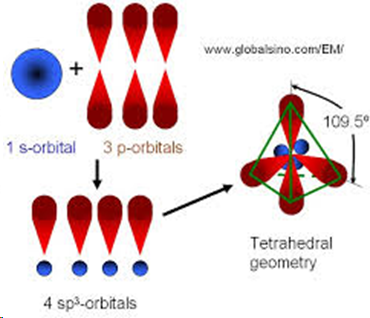
sp3 hybridisation
occurs when three p orbitals and one s orbital hybridise to form four sigma bonds, tetrahedral and has bond angles of 109.5 degrees
sp2 hybridisation
three p orbitals and one s orbital hybridise to form three hybrids and one unhybridised p orbital (which overlaps forming a pi bond), trigonal planar with bond angles of 120 degrees
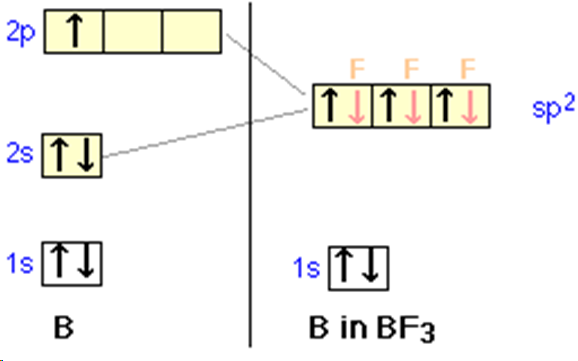
sp hybridisation
three p orbitals and one s orbital hybridise to form two hybrids and two unhybridised p orbitals (which overlap sideways forming two pi bonds), linear with bond angles 180 degrees
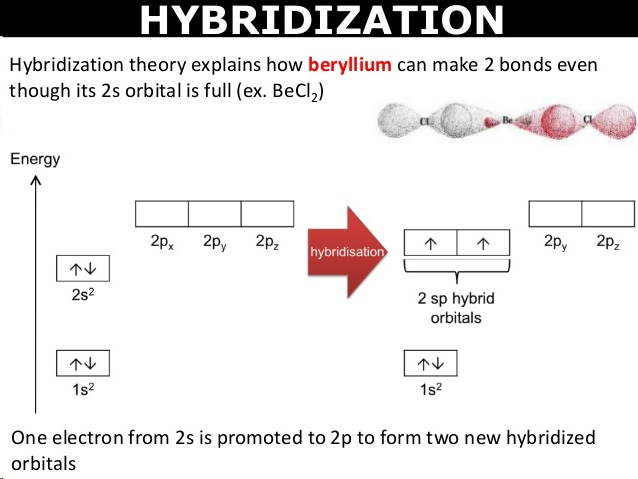
metallic bonds
electrostatic attractions between a lattice of cations and a sea of delocalised electrons
strength of metallic bonding
depends on size (greater positive charge = stronger bond), radius of metal ion (decrease size = stronger bond), no. mobile electrons (more mobile electrons = stronger)
metallic bonding strength/length
delocalised electrons give rise to intermediate bond strength and length
high
if stress is applied to a metal, planes of atoms slide over each other because of delocalised electrons, so metals have ______ malleability
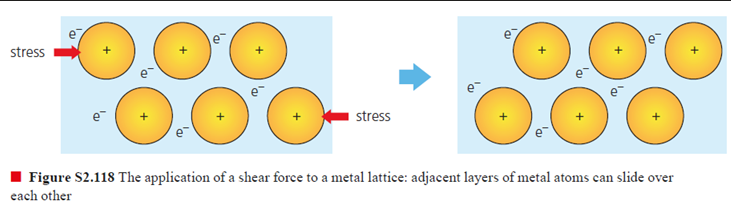
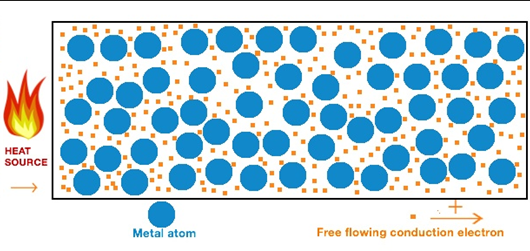
high
when heat is applied to a metal, the kinetic energy of the electrons increases, they move through to cold regions of the lattice, so metals have _______ thermal conductivity
high
if a voltage is applied across the ends of a metal sample, the delocalised electrons flow towards the positive electrode, so metals have _______ electrical conductivity
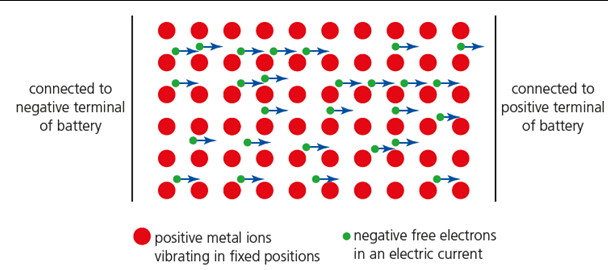
high
lots of energy required to break metallic bonds, influenced by size of positive charge on ion, radius of ion, no. mobile electrons, so metals have ______ melting points
high melting point
because of the greater number of electrons from the d-sublevel being involved in metallic bonding in addition to the e-electrons, transition metals have stronger metallic bonding, which leads to a _________
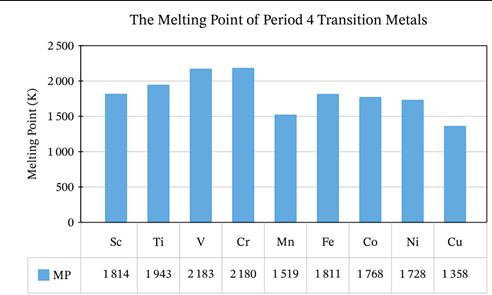
good conductors
because of their loosely bound valence electrons, partially filled d orbitals, and closely packed atomic structure, transition metals are ________
alloys
mixtures of a metal/metal or metal/non-metals (solid solutions), occurs when they are mixed together in a molten state and solidify with ions of of the different metals scattered throughout the lattice
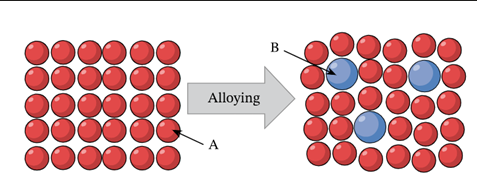
substitutional alloy
atoms of one metal are substitutes by atoms of another metal
interstitial alloy
different metal occupies interstitial spaces in the lattice structure
common alloys
steel, stainless steel, brass, bronze, pewter, sterling silver
van arkel - ketelaar triangle
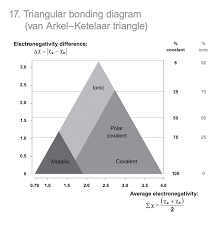
x = (z1 + z2)/2
average electronegativity formula