CH165
1/274
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
275 Terms
what does thermodynamics tell us/not tell us
tells us about heat, work and temperature and their relation to energy, entropy and the physical properties of matter and radiation
doesn’t tell us about timescale or speed of processes or what the pathways are
what variables characterise an equilibrium
temperature
pressure
volume
amount of materials (moles)
what is an open system
can exchange matter and energy/heat
what is a closed system
can’t exchange matter but can exchange energy/heat
what is an isolated system
can’t exchange matter or energy/heat
what are extensive properties and give examples
a property that changes if a different amount of substance is examined
mass
volume
energy
entropy
heat capacity
what are intensive properties and give examples
property is independent of the amount of substance
density
temperature
specific heat capacity (per kg)
molar heat capacity (per mole)
equation for pressure
p = F/A
p = pressure
F = force
A = area
what are the 4 units of pressure and their conversions
Pa
Pa = Nm-2
atm
1 atm = 101,325 Pa
depends on weather/altitude
bar
1 bar = 100,000 Pa (x105)
1 bar = pressure in standard state
Torr
Torr = mmHg
1 Torr = 1/760 atm
conversion between oC and K
0 oC = 273.15 K
what is the state equation for temperature, volume, moles and pressure
pV = nRT
how are p & V related when T & n are fixed
p ∝ 1/V
how are T & V related when p & n are fixed
T ∝ V
how are p & T related when V & n are fixed
p ∝ T
how are n & V related when T & p are fixed
n ∝ V
equation for Boltzmann constant kB
kB = R / NA
kB = Boltzmann constant
R = 8.314 JK-1mol-1
NA = Avogadro number
describe ideal gas and when it is a good model
ideal gases:
all collisions are perfectly elastic
no interaction between particles
point like particles
good model when gas is low pressure and high temperature
equation; pV/nRT = 1
how do real gases differ from ideal gases
attraction between particles, making compression easier
particles repel each other when pushed too close together
particles have finite volume
at low temp, gases condensate and form liquid
equation becomes pV/nRT = Z
Z = compression factor
what does the compression factor suggest
when Z ≈ 1, closer to an ideal gas
when Z < 1, particles attract each other
when Z > 1, particles repel each other
what is kinetic theory
a classical model describing the microscopic behaviour of gas particles and explaining macroscopic properties
point like particles
random movement
no interactions between particles except perfectly elastic collisions
kinetic energy equation
Ek = ½ mv2
equation showing pressure exerted by particles colliding to the wall of a container
pV = 2/3NEk = 2/3N(1/2mv2)
N = number of particles
equation derived from ideal gas law to connect microscopic properties to temperature
Ek = 3/2kBT
what can you calculate with kinetic theory
transport properties and diffusion in gases
frequency of particle collisions
distance travelled between collisions
reaction rates in gases
what happens when there is a mixture of ideal gases
total pressure = sum of contributions from each gas
partial pressure = pressure of that gas if it alone occupied the entire volume of the original mixture at the same temperature
pi = ptotalxi
internal energy definition
the sum of all forms of microscopic energy in the system
work definition
the energy transfer from the system to the surroundings, by a mechanism through which the system can spontaneously exert macroscopic forces on its surroundings
examples of thermodynamic work
electrical work
gravitational work
magnetic
what is volumetric work and what is the equation
when pushing a piston, the gas compresses inside the piston
work is done against a force to move an object by distance dx
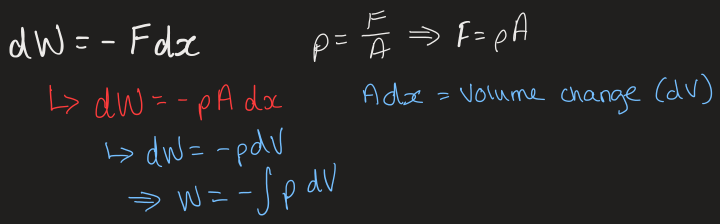
reversible work definition
no clear direction of the process, as the piston is in equilibrium in each step and at each step, work is done against a different force
irreversible work definition
clear direction of the process, the work is done against a constant external pressure
irreversible volume change equation
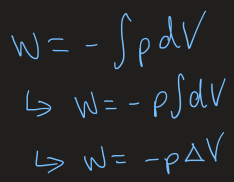
reversible volume change equation
at constant T, can change the volume of a piston reversible
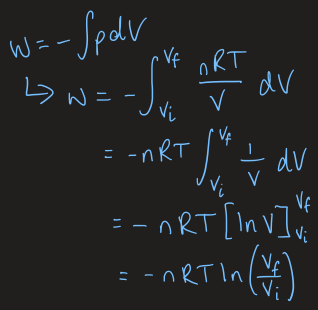
endothermic definition
heat flows into the system
Q > 0
temperature of system increases
temperature of surrounding decreases
exothermic definition
heat flows out of the system
Q < 0
temperature of system decreases
temperature of the surrounding increases
what is the first law of thermodynamics
the energy of a closed system is constant, unless it is changed by transfer of heat or performance of work
equations of W, Q, U when a system is isolated
internal energy cannot change
W = 0
Q = 0
ΔU = 0
equations when no work can be done by a system as it is at constant volume/cannot do electrical work
any change in temperature so Q completely describes the change in internal energy
dV = 0
W = 0
ΔU = Q
equations relating to internal energy when V is constant
no work can be done by a system so any change in T and Q completely describes the change in internal energy
dU = dQ
heat capacity definition
a systems ability to absorb heat as heat
increase in T is different for different materials
(differential) equation to show heat capacity
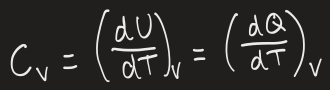
simplified equation when heat capacity is in a narrow T range so doesn’t depend on it
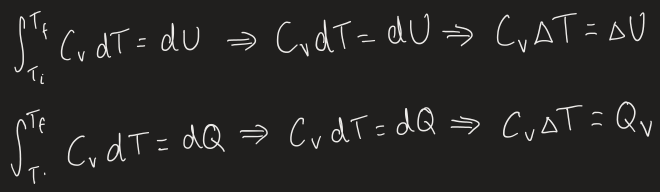
units and symbol for heat capacity
CV
JK-1
units and symbol for molar heat capacity
CV,m = CV/n
JK-1mol-1
units and symbol for specific heat capacity
cV = CV/m
JK-1kg-1
equation for constant volume heat capacity for ideal gases
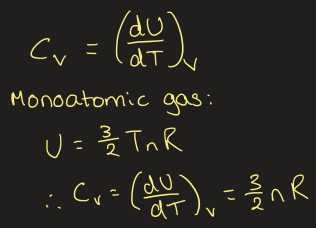
what is the molar heat capacity of the ideal gas
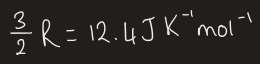
equation for constant volume heat capacity for diatomic ideal gas
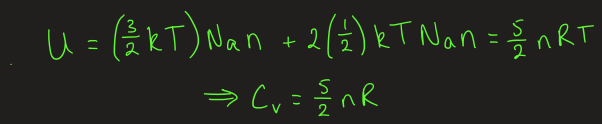
molar CV of diatomic ideal gas

what happens when heat is applied to a substance at constant volume
heat supplied to the system will increase the internal energy of the system by the same amount
ΔU = Q
what happens when heat is applied to a substance with constant pressure
heat supplied to the system will increase the internal energy of the system, but some of it will be used to change the volume
ΔU < Q
enthalpy definition
a change in enthalpy is equal to the heat energy supplied to a system under constant pressure
enthalpy equations
ΔH = Qp
→ H = U + pV
what happens if work is done to the system as it heats (and equations)
the internal energy gained will be greater than the amount of heat
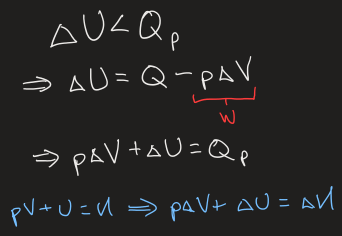
what happens when heat is released/absorbed during a process
if heat is released, ΔHsystem < 0
if heat is absorbed, ΔHsystem > 0
standard state definition
the most stable form of a substance at 1 bar pressure and a specified temperature
in terms of enthalpy, what is defined as the zero point
standard enthalpy of formation of pure elements in their standard states
what is Hess’s law
the standard reaction enthalpy ΔrH is the sum of the individual reactions into which the overall reaction can be separated
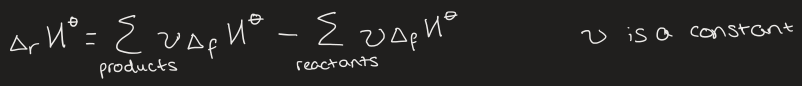
specific heat capacity defintion and equation
heat capacity for a material of specific mass
cp = Cp/m
diagrams + equations for heat capacity as internal energy vs temperature and enthalpy vs temperature

equation for heat capacity at different temperatures

heat capacity equation if assuming heat capacity is independent of temperature
ΔH = CpΔT
heat capacity equation if assuming it is dependent on temperature
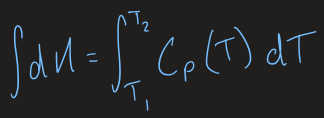
what happens in an adiabatic process and when can it be assumed to be true
no heat or mass is transferred between the system and its surroundings
true for fully isolated systems or when a process is so fast that we can assume that no heat could have been transferred in or out of the system
what is an isobaric process and what equations relate to it
a process occurring at constant pressure
dp = 0
W = -pΔV
dU = dQ + dW
cp = ΔH/ΔT
what is an isochoric process and what are the relating equations
a process occurring at constant volume
dV = 0
W = 0
dU = dQ
cV = ΔV / ΔT
what is an isothermal process and what are the relating equations
a process occuring at constant temperature
dT = 0
W = -∫p dV
dU = dQ + dW
what are the equations relating to adiabatic processes
dQ = 0
W = ΔV
dU = dW
c = 0
equation for entropy
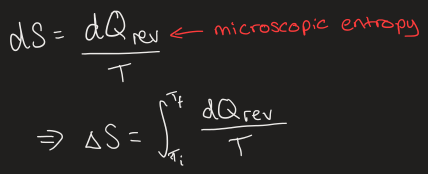
what causes entropy change
more disorder = larger entropy
solid < liquid < gas
increased chemical complexity = larger entropy
NaCl < MgCl2 < AlCl3
softer metal = larger entropy
diamond < graphite < lead
equation for universal entropy
ΔSuniv = ΔSsurrounding + ΔSsystem
ΔSuniv > 0
what happens to entropy as T → 0 K
entropy change tends towards 0
any pure substance in a perfect crystalline structure, at the absolute 0, has 0 entropy
what is Helmholtz energy equation and what is the condition
constant volume
ΔA = ΔU - TΔS
A is Helmholtz free energy
what is Gibbs energy equation and what is the condition
constant pressure
ΔG = ΔH - TΔS
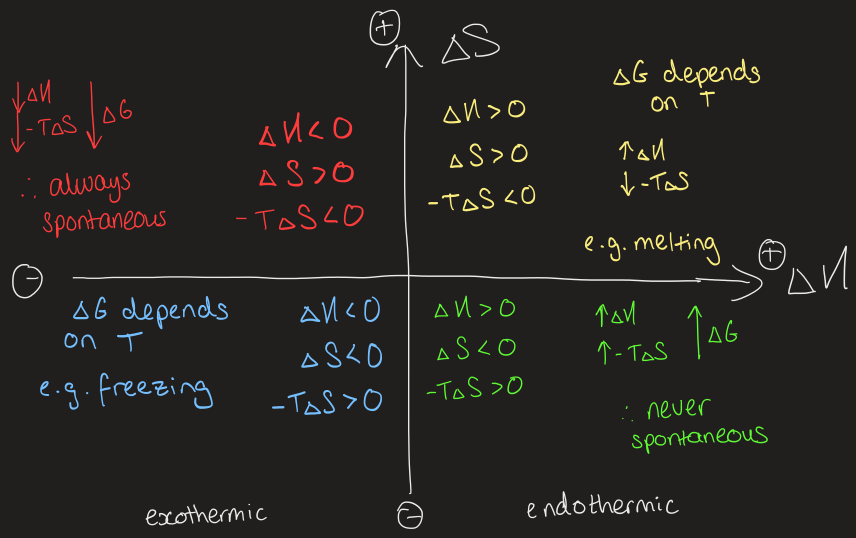
what does ΔG tell you
if ΔG < 0 then the process is favourable and will occur spontaneously
if ΔGB < ΔGA then B is more favourable
what is an endergonic process
non-spontaneous process where ΔG > 0
what is an exergonic process
spontaneous process where ΔG < 0
draw a diagram to predict spontaneity using ΔS and ΔH
equations for calculating the standard Gibbs energy of the reaction
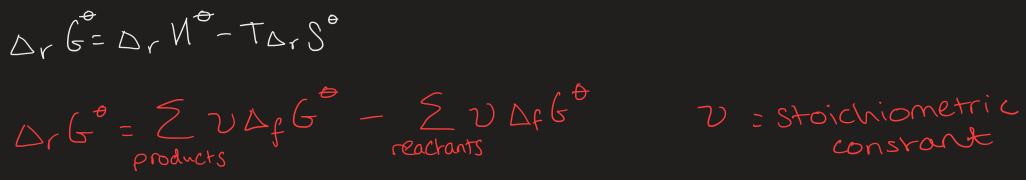
equations for calculating the standard Gibbs energy of formation
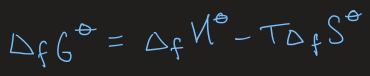
natural variables and equation for state function U
S, V
dU = TdS - pdV
natural variables and equation for state function S
U, V
not possible to form equation
natural variables and equation for state function H
S, p
dH = TdS + Vdp
natural variables and equation for state function G
p, T
dG = Vdp - SdT
natural variables and equation for state function A
V, T
dA = -pdV - SdT
what do each of these letters mean:
T
V
p
n
x
U
W
Q
H
S
G
F
A
K
R
c
μ
temperature
volume
pressure
amount of matter
mole fraction
internal energy
work
heat
enthalpy
entropy
Gibbs energy
force
Helmholtz energy
equilibrium constant
universal gas constant
heat capacity
chemical potential
phase definition
a form of matter that is microscopically uniform throughout in both chemical composition and physical state
vaporisation equations
enthalpy change
entropy change
ΔvapH = Hgas - Hliquid > 0
ΔvapS = Sgas - Sliquid > 0
condensation equations
enthalpy change
entropy change
ΔcondH = Hliquid - Hgas < 0
ΔcondS = Sliquid - Sgas < 0
effect of temperature at vaporisation
at bp
at low T
at high T
ΔvapG = ΔvapH - TbΔvapS = 0
so ΔvapH = TbΔvapS
so Tb = ΔvapH / ΔvapS
GL < Gg
liquid is more favourable
condensation occurs
GL > Gg
gas is more favourable
vapourisation occurs
what is Trouton’s rule
for many liquids the entropy of vaporisation is almost the same, around 85 J/K
effect of pressure on vapourisation
higher pressure = smaller volume of gas so less freedom to move = smaller entropy do gas less favourable
what is Gibbs free energy equation for an ideal gas if pressure is changed

equation for Gibbs energy change compared to standard state
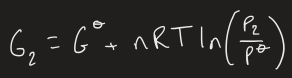
boiling curve definition
the group of p-T points, where liquid and gas are in equilibrium so they coexist
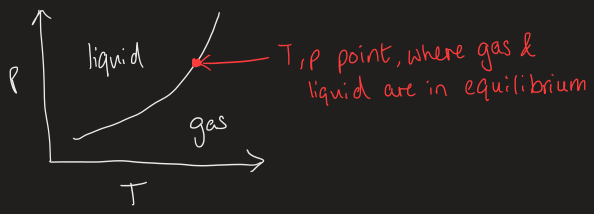
what happens to the phase when you increase pressure/temperature
as you increase pressure, the pressure will stop and remain constant as liquid forms so there is less gas
as temperature increases, the increase in heat stays constant but change in T = 0 as all heat is supplied to phase transition
equations used to find value of vapour pressure at a given temperature
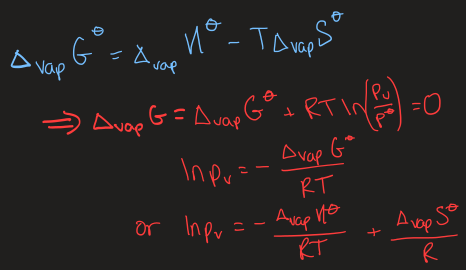
the Clausius-Clapeyron equation
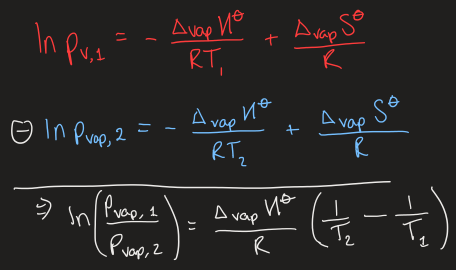
at fixed volume, how can you tell whether a substance is liquid or gas
if p < pvap then substance is a gas
what happens when you add more gas to a fixed volume container
it will immediately condense