Elements of Life
1/38
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Structure of Atom
Positively charged nucleus containing protons and neutrons with negatively charged electrons orbiting around it, in shells.
Electrons make up the most volume of atoms, atom is mostly empty space.
Relative mass of electrons
Negligible
Atomic number
Number of protons in the nucleus of an atom
Why do all atoms of the same element have same atomic number?
Same number of protons in nucleus
Mass number
Number of protons and neutrons in the nucleus of an atom
Ion
Has different numbers of protons and electrons (protons = electrons)
1- Dalton’s model
Early 19th century - solid spheres and the smallest particles of an element. Elements made from different spheres.
2- J J Thompson
1897 - Plum Pudding model.
Discovery of electrons and its mass + charge.
Positively charged sphere with negatively charged electrons embedded into it.
3- Rutherfold
1909 - Nuclear model.
Discovered nucleus
Gold leaf experiment - Fired alpha particles at thin sheet of gold. Expected most to be deflected very slightly but most past straight through + some deflected back.
Positive nucleus in mainly empty space with cloud of electrons
4- Chadwick
Discovered neutrons.
Fired alpha particles at a thin sheet of Beryllium
5- Bohr
Electrons in fixed energy orbitals.
When an electron moves between shells, EMR is emitted or absorbed.
Each shell can only hold a fixed number of electrons.
6- Quantum model
Electrons doesn’t have same energy in shells.
Contain subshells.
Isotopes
atoms that have the same number of protons but a different number of neutrons.
Have the same chemical properties as they have the same electron configurations.
Have slightly different physical properties as they depend on the mass of an atom
Relative atomic mass (Ar)
The average mass of one atom of the element, taking into account all the isotopes and their abundances, relative to carbon-12 = 12g.
Relative isotopic mass
average mass of an isotopic atom of an element, relative to 12g of carbon-12.
Relative molecular mass (Mr)
The average mass of a molecule or formula unit, relative to carbon-12 = 12g
One mole of a substance
contains the same number of particles as there are atoms in 12g of carbon-12.
= is the Mr in grams.
= 6.02 x1023 (Avogadro constant, NA)
Empirical formula
The smallest number ratio of atoms in a molecule.
Molecular formula
The actual number of atoms of each element in a molecule
Hydrated solids
Compounds containing water molecules incorporated into them; water of crystallisation
% yield formula
% yield= (actual moles of product)/(theoretical moles of product) ×100
Why % yield isn’t 100%
Loss from unreacted chemical reagents
Mass lost in transferring materials with equipment
Reversible reaction so products turn back into reactants
Mass lost if gas products escape into surroundings
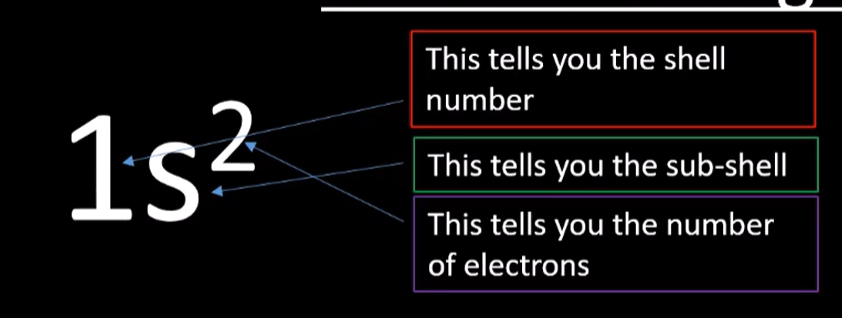
Electronic structure
Electrons exist in shells which contain subshells - s,p,d,f
Each subshell consists of different number of atomic orbitals – s=1, p=3, d=5, f=7.
Atomic orbital
A region of space in an atom where there is a high probability of finding the electron.
Each atomic orbital can hold up to 2 electrons – have opposite spin.
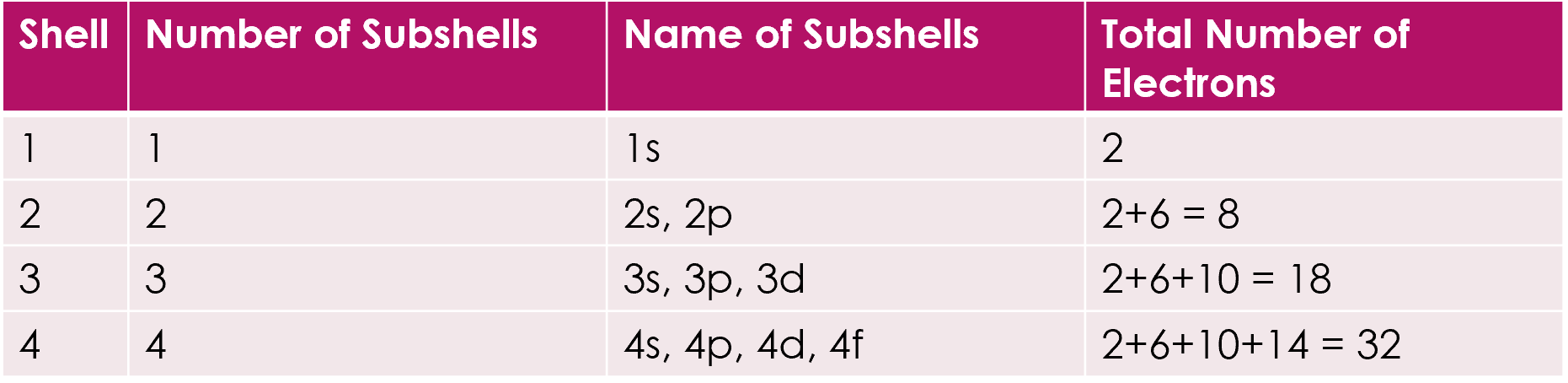
Shells and subshells
1 = 1s = 2e
2 = 2s, 2p = 8e
3 = 3s, 3p, 3d = 18e
4 = 4s, 4p, 4d, 4f = 32e
Orbitals
s-orbital is spherical-shaped
p-orbital is dumb-bell shaped
The closer the average distance of an electron to the nucleus, the lower its energy
An electron will occupy the lowest energy subshell available
Ionic bonding
Forms ions when electrons are transferred between atoms
Strong electrostatic attraction between positively and negatively charged ion.
Forms giant ionic lattice
Physical properties of ionic compounds
Solids at room temperature with a high melting point – very strong electrostatic attractions between opposite ions in the lattice need a lot of thermal energy to break.
Most are water soluble – water molecules are polar which form attractions to the charged ions.
Conduct electricity when molten or in solution – ions are free to move and carry charge.
Covalent bond
Shared pair of electrons.
Dative bond
Covalent bond in which both electrons came from the same atom
Giant covalent structures vs simple molecules
have stronger electrostatic attraction than simple covalent molecules
Physical properties of simple covalent molecules
low melting + bp, weak intermolecular forces so little thermal energy required
Can’t conduct electricity - no overall charge
Most insoluble in water
Physical properties of giant covalent lattices
High melting point - strong intermolecular forces in covalent bonds
Insoluble in any solvent
Can’t conduct electricity except graphite
Extremely hard from strong bonds
Good thermal conductors - vibrations travel easily through lattice
Metallic structures
Positively charged metal ions lattice surrounded by a sea of delocalised electrons with strong attraction.
Physical properties of metals
High melting point + solid at room temperature - strong electrostatic forces
Group 2 higher mp than group 1 as ions has higher charge so stronger electrostatic attractions
Conducts electricity - delocalised electrons are free to move and carry charge
Ductile, metal ions slide over each other as no bonds holding them together
Insoluble
Shape of molecules
Sets of electron pairs around a central atom repelling to get as far apart as possible.
Lone pairs repel more than bonding pairs
Elements in the same period have
the same number of electron shells occupied
Elements in the same group have
the same number of electrons in their outer shell.
similar physical and chemical properties
Mp of metals increase across the period because
stronger metallic bonds as metal ions have increasing number of delocalised electrons and smaller ionic radius (pulled closer from more protons) so higher charge density thus stronger electrostatic attractions.