AMI - Lecture 7 - Autoantibodies in infection and inflammation
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
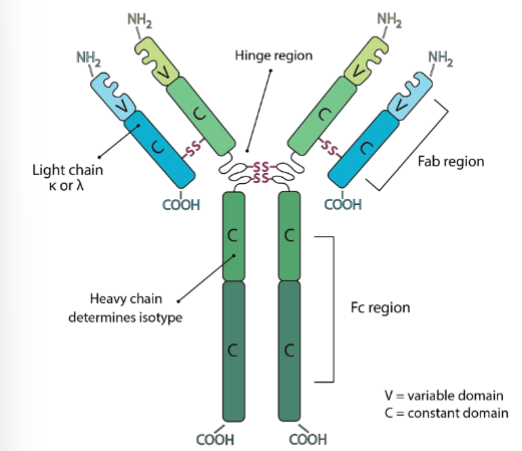
explain the following terms; Fc, Fab, hinge, light + heavy chain
Fc region → immune cells bind here, constant domain
Fab region → where ag binds, consists of constant + variable region
hinge → connects Fc + Fab
heavy chain → determines isotype
What are the different types of ab, explain the different IgG
IgG is the most prevalent in the serum
IgG1, most common
IgG2, stiffer hinge region, activates complement and is proteolytic
IgG3, long hinge region = flexible, activates complement + proteolytic (better than IgG2).
IgG4, binds 2 different ag, no immune response but can stop interactions, anti inflammatory ab, functionally monovalent
IgD unknown
IgE role in allergic reaction, asthma, parasites
IgM is the first Ab produced after infection, pentameric, activation complement
IgA can be monomeric in the blood, but can also be secretory
explain more about the ab IgA
produced most of all ab per day
key antibody involved in mucosal immunity
IgA is transported to mucosal site (transported across epithelium), then released as secretory IgA
functions;
prevent pathogen entry by binding pathogens + neutralizing
IgA coated ag taken up by M cells and delivered to ag presenting cells in Peyer’s patches
IgA can be anti inflammatory → promote anti-inflammatory cytokines, prevent overactivation of immune responses
the 3 functions of antibodies
neutralizes pathogens at mucosal site
activates complement → MAC
opsonizes pathogens → coats them with ab so they are more recognizable, will be phagocytosed
activating versus inhibitory fc receptors
fc receptors are found on the surface of immune cells → bind to fc region of antibodies
fc receptors can be activating or inhibitory
activating → lead to phagocytosis, cytokine release
inhibitory → suppress immune activation to prevent excessive inflammation
the balance will determine the outcome of the cell
What are autoantibodies
antibodies produced by the immune system that mistakenly target the body's own tissues, cells, or proteins
common diseases;
Systemic lupus erythematosus (SLE): Anti-dsDNA antibodies.
Rheumatoid arthritis: Rheumatoid factor (RF) and anti-CCP antibodies.
Type 1 diabetes: Anti-GAD antibodies.
systemic lupus erythematous
autoimmune disease
reacts to DNA
symptom; butterfly rash on face
impaired clearance of apoptotic cells in SLE
impaired clearance of apoptotic cells
accumulation secondary necrotic cells (SNEC)
release of necrotic debris
autoag activate immune response (BC)
break of BC tolerance, continuous exposure of autoab in germinal centres leads to production of autoab
NETosis and immune complex formation
NETosis → process where neutrophils release chromatin and antimicrobial proteins to trap pathogens. Normally DNAse clears NETs to prevent excessive accumulation
in SLE → defects in DNAse → uncleared NETs → nuclear material exposed to environment such as DNA, histones → these act as autoantigens, trigger immune response → autoantibodies bind to autoantigens, form immune complexes → immune complexes + uncleared NETs result in activation DC and production of interferon alpha → resulting in organ damage
autoab in rheumatoid arthiritis
chronic auto immune disease
inflammation of joints → infiltration of neutrophils in synovial fluid → destruction of cartilage and bone
RF-IgM and anti-CCP-IgG can be used to diagnose
RF-IgM → rheumatoid factor → is an antibody against the Fc tail of IgG. (ab against ab).
when binding happens immune complexes formed → taken up by neutrophils → induce NETs → tissue damage and inflammation
anti-CCP-IgG → anti-cyclic citrullinated peptide (modified forms of self proteins in RA).
increased IgA in RA
increased IgA autoantibodies in RA correlates with worse disease outcomes
neutrophils in RA overactivated → NET release mediated with IgA
in RA overactivated osteoclasts → these break down bone. when IgA activated monocytes were cultured on bone matrix resulted in large hole in bone.
Linear IgA bullous disease (LABD)
immune system produces IgA autoab against collagen 17 (protein that anchors epidermis to dermis)
neutrophils recruited to this site → tissue inflammation
challenges in studying→ mice do have IgA but no fc receptor
in LABD research → mouse model with knock in human IgA directed against mouse collagen 17
fluorescent neutrophils injected in ear (very thin, visible under microscope) showed high influx of neutrophil into tissue.
explain monoclonal ab production
mouse injected with ag to stimulate B cell response (ab against injected ag)
myeloma cells (cancerous B cells that divide indefinitely) are fused with B cells from mouse (hybridoma)
these fused cells (hybridomas) grow in drug containing medium only the hybrid cells live
hybridomas are selected that produce ag specific ab
this hybridoma is cloned
These ab used in research
hoe are mice derived ab ultimately changed to be used in human therapeutics (3 techniques)
chimeric ab → 75% human 25% mouse, variabel region (Fab, where ag binds) is from mice, rest of ab from human, very successful in clinic
production human antibodies (less anti- animal reaction);
humanization → graft the complimentary determining region (CDR), these are key ag binding site from mouse, onto human framework
HuMab mice → immunoglobulin genes knocked out in mice and replaced with human Ig genes. mice start producing fully human ab
how do ab work in therapeutics (autoimmune disease)
bind/block cytokines → no signal transduction
rituximab → anti CD20 (B surface marker). it depletes B cells, is used to treat B cell malignancies and used in autoimmune disease.
Bevacizumab → anti VEGF (vascular endothelial growth factor). mab used in cancer therapy.
checkpoint inhibitors → anti PD1, removes the break from immune system, resulting in effective T cells. can cause side effects that immune system starts attacking healthy tissue
FcR blocking → used to reduce tissue damage. Prevents immune cells from attacking healthy tissue
explain the 6 ways mab work
enhancing cancer treatment → increased sensitivity to radiation and chemotherapy
herceptin for breast cancer
blocking growth factor receptors → antagonistic ab, stops tumors from receiving growth signals.
EGFR → tumor uses EGF to grow. can block this receptor, tumor can not grow. but sometimes patients have mutation in EGFR then this treatment does not work.
complement activation → requires at least 2 IgG molecules
MAC
fc receptor mediated effects → several effects;
NK cells → also bind to tumor, attract NK cells. KIR receptors on NK cells regulate inhibitory or activating signals, mab increase the activating signals
MQs result in phagocytosis, Kupffer cell (specialized MQ in liver) effectively clear circulating tumor cells. (don’t always fully kill tumor leading to gradual death)
neutrophils → immune repressive in cancer patients.
cetuximab (IgG ab) → not very effective
cetuximab (IgA ab) → works very well
G-CSF → promote pro inflammatory environment, increase numbers at tumor site, neutrophils secrete chemotactic stimuli and attract other immune cells
trogocytosis → neutrophils mechanically pull of parts of tumor
explain how mab therapy can be improved
changing isotype (IgG, IgG4)
improving fc receptor binding
using ab as drug carriers (ab drug conjugates (ADC))
IMPORTANT → CD8 T cells do not express fcR → no direct affect of mab therapy that rely on fcR mediated mechanisms
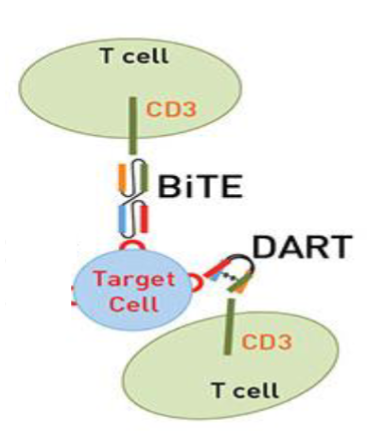
explain BiTEs and DARTs
BITEs + DARTs → guide T cells directly to tumor cells by binding both CD3 and tumor associated ag
thus bypass the need for fcR
BITEs → target CD3 on T cells and CD19 on B cells
DARTs → works the same but with great specificty and bind multiple targets
both have short half lives → continuous infusion needed
chimeric antigen receptor T cells (CAR T)
genetically engineered T cells designed to recognize and destroy specific cancer cells
based on the antigen recognition domain