Alkenes
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
What is the functional group of alkenes
C=C (carbon carbon double bond)
2
New cards
What is the general formula for alkenes?
CnH2n
3
New cards
Why are alkenes classified as unsaturated hydrocarbons?
They contain one or more C=C double bonds
4
New cards
What are the first four alkenes?
Ethene
Propene
Butene
Pentene
5
New cards
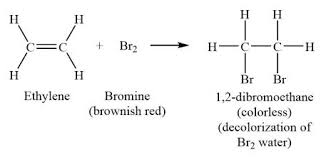
Describe the reactions of alkenes with bromine to produce dibromoalkanes and what type of reaction it is.
Addition reaction
alkene + bromine —> dibromoalkane
orange —> colourless
removes the carbon carbon double bond
bromine = Br2
6
New cards
How can bromine water be used to distinguish between an alkane and alkene?
Alkene reaction : orange —> colourless
Alkane reaction : remain orange